Xeroderma Pigmentosum, Complementation Group C
A number sign (#) is used with this entry because xeroderma pigmentosum complementation group C (XPC) is caused by mutation in the XPC gene (613208) on chromosome 3p25.
DescriptionXeroderma pigmentosum is a genetically heterogeneous condition characterized by increased sensitivity to ultraviolet (UV) irradiation and increased risk of skin cancer resulting from a defect in DNA repair. XPC is the most common form of XP in the white population, accounting for over a third of all cases in this group (review by Li et al., 1993).
For a general discussion of xeroderma pigmentosum, see XPA (278700).
Clinical FeaturesLynch et al. (1984) suggested that complementation group C patients may be particularly prone to malignant melanoma.
Li et al. (1993) identified 2 patients with XPC confirmed by genetic analysis (613208.0003). Cell lines from these patients were the least sensitive to UV-irradiation compared to 4 other cell lines, and exhibited a near-normal level of XPC mRNA. Clinically, the proband was diagnosed with XP at birth and was rigorously protected from sunlight from that time; as of 13 years of age, the patient had not exhibited any malignant neoplasms. However, an older brother with XP began to develop tumors by age 13. Like the vast majority of XPC patients, this patient did not exhibit neurologic complications.
Chavanne et al. (2000) reported 12 patients with XPC. None showed neurologic abnormalities. DNA repair activity ranged from 10 to 20% of normal.
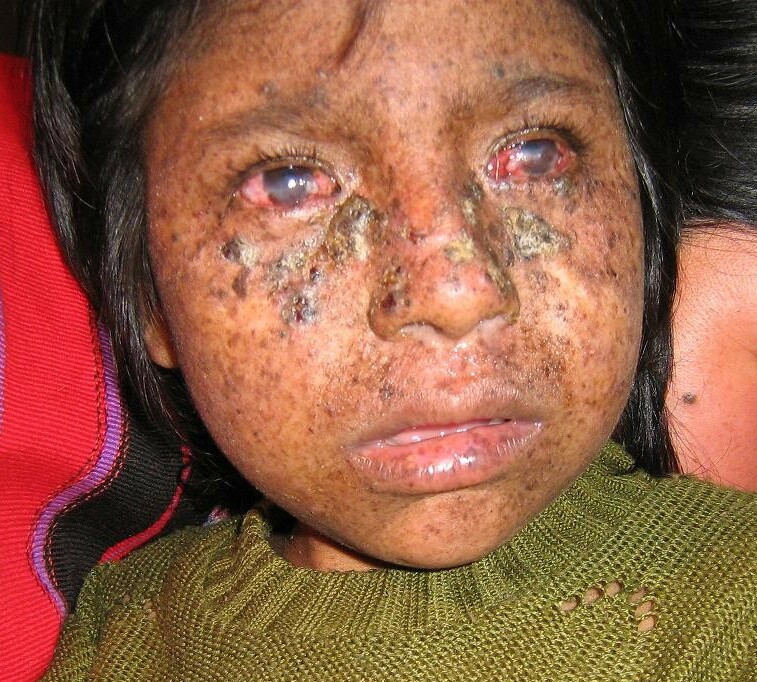
Gozukara et al. (2001) reported a Turkish family with severe XPC confirmed by genetic analysis (R579X; 613208.0007). The 8-year-old boy had abnormal skin lesions by 1 year of age, and developed typical freckling on sun-exposed areas with atrophy, telangiectasia, hypopigmentation, and actinic keratoses. He had multiple skin cancers and died at age 10 years. There were no neurologic abnormalities. His 5-year-old sister developed abnormal skin lesions at age 6 months and squamous cell carcinoma on her face at age 2 years. A cousin was also reportedly affected. The DNA repair level was 12 to 16% of normal.
Khan et al. (2004) reported 2 consanguineous Turkish families with XPC. In the first family, a 20-year-old male and his 16-year-old sister were both severely affected. They developed skin lesions at 3 years of age. Both had cutaneous atrophy, telangiectasia, actinic keratoses, and multiple skin cancers including squamous cell carcinomas, basal cell carcinomas, and melanomas. In the second family, 3 sisters, aged 20, 18, and 11 years, were more mildly affected. Skin lesions began at age 3 to 5 years. They had freckling but no skin atrophy, telangiectasia, or actinic keratoses. The oldest sister had a squamous cell carcinoma excised from her face at age 12 years. The other sisters did not have skin cancer.
Clinical Variability
Hananian and Cleaver (1980) reported an unusual patient with XPC diagnosed by cell complementation studies who had neurologic symptoms and features of systemic lupus erythematosus (SLE; see 152700).
Khan et al. (1998) reported a 4-year-old boy of Korean ancestry with XPC. The phenotype was characterized by sun sensitivity and multiple cutaneous neoplasms, as well as unusual neurologic features, including hyperactivity and autistic features. Other typical XP neurologic abnormalities were not preset. In addition, laboratory studies showed persistently low levels of glycine. Hyperactivity diminished with oral glycine supplements. Genetic analysis identified a mutation in the XPC gene (613208.0005).
PathogenesisXP patients are susceptible to UV-induced freckling and malignant skin changes, whereas Cockayne syndrome (see, e.g., 216400) patients do not have increased susceptibility to these lesions. Seguin et al. (1988) studied the frequency of ultraviolet light-induced chromosomal breaks in lymphoblastoid cell lines from patients with Cockayne syndrome and from patients with xeroderma pigmentosum. Cells established from patients with either disorder had the same abnormal increase in the number of induced aberrations. However, the authors concluded that the frequency of UV-induced breakage in the chromosomes of xeroderma pigmentosum group C cells does not explain the susceptibility of these patients to sunlight-induced skin cancer.
Molecular GeneticsLi et al. (1993) identified changes in the XPC gene (see, e.g., 613208.0001-613208.0004) in 5 XPC cell lines. In 4 of them, Northern blot analysis of RNAs demonstrated subnormal levels of the XPC transcript, whereas the fifth exhibited a near normal level.
In affected members of 2 unrelated but consanguineous Turkish families with XPC, Khan et al. (2004) identified 2 different splice site mutations in the XPC gene (613208.0008 and 613208.0009), respectively. RT-PCR of cells from the severely affected patients showed a short mRNA band and no detectable wildtype band. In contrast, cells from the more mildly affected patients had an mRNA band of shorter size and 1 of normal size. These findings correlated with disease severity.
Cleaver et al. (1999) reviewed mutations in the XPC gene.
Population GeneticsBen Rekaya et al. (2009) reported a high frequency of XPC in Tunisia. They reported 14 affected Tunisian families, 12 of which were consanguineous. Age at onset ranged from 1 to 96 months and the average age of the patients was 11 years. Clinical features included photophobia and skin tumors, including basal cell carcinoma, squamous cell carcinoma, and malignant melanoma. None of the patients had neurologic abnormalities. Genetic analysis showed that all patients carried the same homozygous 2-bp deletion (1744delTG; 613208.0010) in the XPC gene. Haplotype analysis indicated a founder effect.
HistoryA ninth complementation group (XPI) described by Fischer et al. (1985) was withdrawn by Bootsma et al. (1989). The initial classification had been done on the basis of studies of 1 cell line only. Study with a second biopsy from both of the 2 sibs showed that the cells behaved exactly like those of the XPC complementation group. Bootsma et al. (1989) suspected that the original cell strain was contaminated with another XP cell strain, at least at the stage when complementation experiments with XPC fibroblasts were performed. If these contaminating cells belong to an XP complementation group other than C, one might expect to find complementation in all instances, even with itself. Unfortunately, the latter control experiment was not performed in the original study.