Cherubism
A number sign (#) is used with this entry because of evidence that cherubism is caused by heterozygous mutation in the SH3BP2 gene (602104) on chromosome 4p16.
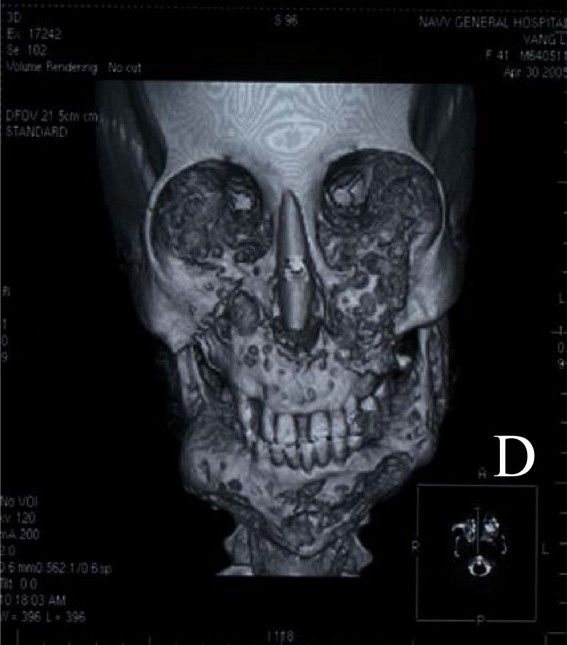
Clinical FeaturesSwelling of the lower face begins around the third or fourth year of life and progresses until the late teens. The enlargement may be exaggerated by enlargement of submandibular lymph nodes. X-ray reveals multilocular cystic changes in the mandible and maxilla and often in the anterior ends of the ribs. Though clinical swelling usually abates by the third decade, radiographic changes commonly persist into the fourth decade. The condition must be differentiated from Caffey disease (114000) in which the x-ray appearance is different and involvement of the skeleton, e.g., the tibia, is more widespread. It is, like Caffey disease, a benign self-limited condition. The disorder has also been called familial benign giant-cell tumor of the jaw, familial multilocular cystic disease of the jaw, etc. (see Khosla and Korobkin, 1970; Peters, 1979).
Jones (1965), who was the first to describe the entity, pointed out that lack of signs or history in either parent does not exclude the possibility of one's being affected. In one of his cases, the disorder would not have been discovered, or even suspected, were it not that x-rays were made in childhood in a deliberate search for the entity because of its occurrence in other members of the family.
Salinas et al. (1983) reported 2 cases of cherubism with multilocular cystic lesions of the ribs in addition to those of the mandible. In 1 of the patients, biopsy of both the jaw and the rib lesions showed numerous multinucleated giant cells in cellular fibrous tissue.
Quan et al. (1995) described cherubism in association with mental retardation due to mosaicism for expansion and deletion of the FMR1 (309550) CGG repeat, i.e., the fragile X syndrome (300624). Although these were probably independent mutations, Quan et al. (1995) pointed out the peculiarities of the inheritance of cherubism, which has been thought to be an autosomal dominant: twice as many males are affected as females, and whereas penetrance in males is 100%, penetrance in females is only 50 to 70%.
Tiziani et al. (1999) reported a 3-generation family, in which the mandible in males was more severely affected than the maxilla, whereas in females the maxilla was more severely affected. On average, the clinical onset of the disease was earlier in females (5.5 years of age) than in males (10.6 years of age). The description of the 14-year-old female proband with maxillary enlargement that started at the age of 5 included enlarged submandibular lymph nodes.
Stiller et al. (2000) reported 3 affected males (a boy, his father, and his paternal grandfather) with cherubism. The boy also had craniosynostosis of the sagittal and coronal sutures. The father and paternal grandfather had cherubism and clubbing of the fingers; neither had any underlying cardiac or pulmonary problem which could explain their clubbed fingers.
Ahmadi et al. (2003) reported a case of orbital cherubism with visual loss directly attributable to optic neuropathy and macular striae/scarring that resulted from the effect of the orbital fibroosseous tumor pressing on the eye.
MappingTiziani et al. (1999) and Mangion et al. (1999) mapped a cherubism locus to chromosome 4p16. Tiziani et al. (1999) used a genomewide search in a 3-generation family. Three other families affected with cherubism were also genotyped and were mapped to the same locus. The combined lod score was 4.21 at a recombination fraction of zero, and the locus spanned an interval of approximately 22 cM. Using 2 families with clinically, radiologically, and/or histologically proved cherubism, Mangion et al. (1999) also performed a genomewide linkage search and mapped the locus to chromosome 4p16.3, with a maximum multipoint lod score at 5.64. Critical meiotic recombinants restricted the locus to a 3-cM interval between D4S127 and the telomere of 4p.
By linkage and haplotype analysis of 12 families with cherubism, Ueki et al. (2001) refined the cherubism locus to a 1.5-Mb interval between markers D4S127 and D4S115 on chromosome 4p16.
Molecular GeneticsBy sequencing cDNA and genomic DNA from affected and unaffected members from 12 families with cherubism, Ueki et al. (2001) detected point mutations that caused amino acid substitutions in the SH3BP2 gene (602104.0001-602104.0007). All mutations were in exon 9 and affected 3 amino acids within a 6-amino acid sequence (RSPPDG) located 31 to 36 amino acids upstream of the SH2 domain and 205 to 210 amino acids downstream of the SH3-binding domain. Mutations in pro418 (to leu, arg, or his) were the most common and occurred in 8 families. Other mutations resulted in gly420 being replaced by glu or arg and in arg415 being replaced by pro or gln. SH3BP2 lies within a region that is frequently deleted in individuals with Wolf-Hirschhorn syndrome (194190). Haploinsufficiency of SH3BP2 in individuals with that syndrome does not result in cherubism or cherubism-like characteristics. This finding and the clustering of amino acid missense mutations in SH3BP2 support the hypothesis that the mutations in SH3BP2 lead to a gain of function or act in a dominant-negative manner. The onset of the abnormalities of cherubism and their organ-restricted characteristics may be related to dental developmental processes in children, when signals unique to the mandible and maxilla are transmitted through the extracellular matrix, triggered by the eruption of secondary teeth.