Cherubism
Summary
Clinical characteristics.
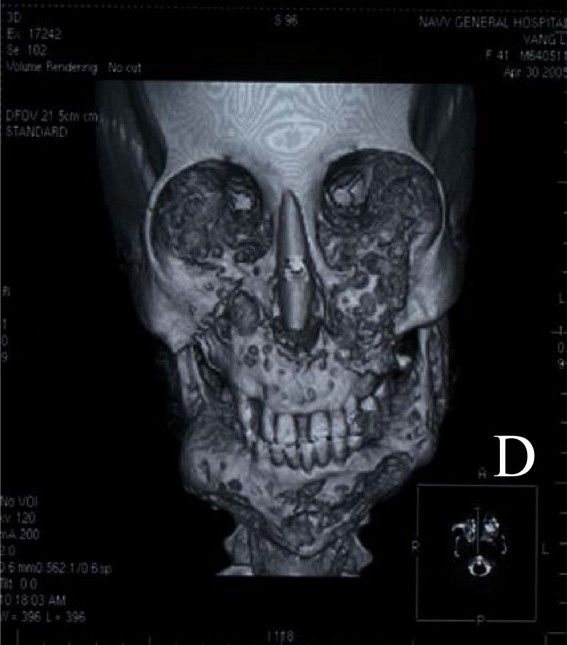
Cherubism is a childhood-onset, autoinflammatory bone disease characterized by bilateral and symmetric proliferative fibroosseous lesions limited to the mandible and maxilla. The enlargement is usually symmetric in nature. The phenotype ranges from no clinical manifestations to severe mandibular and maxillary overgrowth with respiratory, vision, speech, and swallowing problems. In most affected persons, teeth are displaced, unerupted, unformed, or absent, or may appear to be floating in cystlike spaces; malocclusion, premature exfoliation of deciduous teeth, and root resorption have also been reported. The course and duration of the active process of bone destruction varies between affected individuals; the onset is usually in early childhood, and typically new lesions can occur until puberty. Regression of the lesions occurs as they become filled with bone and remodel during the second and third decade of life. By age 30 years, the facial abnormalities associated with cherubism are not usually recognizable and residual deformity of the jaws is rare. Typically, cherubism is an isolated benign condition; the affected person has normal intellectual skills and is without other physical anomalies.
Diagnosis/testing.
Diagnosis is established in a proband with typical clinical, radiographic, and histologic findings and/or a heterozygous pathogenic variant in SH3BP2 identified by molecular genetic testing.
Management.
Treatment of manifestations: Given that cherubism is considered to be a self-limited condition that improves over time, treatment should be tailored to the individual's presentation and the evolution of the disease. Ongoing management by a craniofacial team in a major pediatric medical center is recommended; depending on the severity, surgery may be needed for functional and esthetic concerns.
Surveillance: Long-term follow up with clinical, radiographic, dental, orthodontic, and ophthalmologic evaluations. Annual reviews are indicated while new cysts continue to form or established cysts enlarge; after the disorder becomes quiescent, follow up every two to five years.
Testing of relatives at risk: When the pathogenic variant in the family is known, molecular testing can be used to identify mildly affected relatives who may benefit from early intervention; otherwise, clinical and radiographic evaluations can identify relatives at risk.
Genetic counseling.
Cherubism is inherited in an autosomal dominant manner. The proportion of cases caused by de novo pathogenic variants is unknown because of variable expressivity and reduced penetrance. Each child of an individual with cherubism has a 50% chance of inheriting the pathogenic variant. Prenatal diagnosis for pregnancies at increased risk and preimplantation diagnosis are possible if the pathogenic variant has been identified in the family.
Diagnosis
Cherubism is a childhood-onset, autoinflammatory bone disease characterized by bilateral and symmetric proliferative fibroosseous lesions limited to the mandible and maxilla. The course and duration of the active process of bone destruction varies between affected individuals; the onset is usually in early childhood, and typically new lesions can occur until puberty. Regression of the lesions occurs as they become filled with bone and remodel during the second and third decade of life. No formal clinical diagnostic criteria for cherubism have been published to date.
Some experts consider a tissue biopsy sufficient to confirm the diagnosis. However, since cherubic phenotypes can be mimicked by other jaw tumors requiring different therapeutic strategies, a thoughtful consideration of histologic analysis is warranted [Friedrich et al 2016].
Suggestive Findings
Cherubism should be suspected in individuals with the following clinical, radiologic, and histologic findings.
Clinical findings
- Onset usually between age two and seven years
- Bilateral, symmetric enlargement of the mandible and/or maxilla including coronoids but usually sparing the condyles. Other cranial bones are usually unaffected.
- Swelling of submandibular and cervical lymph nodes (early in the course only)
- Slow progression of the jaw lesions up to adolescence and spontaneous regression typically starting after puberty and extending into the twenties
- Upturned tilting of eyeballs (in advanced stages); rim of sclera visible beneath iris
- Dental abnormalities: congenitally missing second and third molars; premature exfoliation of the deciduous teeth and displacement of permanent teeth secondary to the jaw lesions; malocclusion
Radiographic manifestations typically include bilateral, multilocular, radiolucent areas within the mandible, usually located at the angles and rami. The coronoid processes are commonly involved, whereas the condyles are rarely affected.
- Lesions in the mandible are usually symmetric, whereas those in the maxilla may be asymmetric.
- Imaging typically shows expansile remodeling of the involved bones, thinning of the cortices, and multilocular radiolucencies with a coarse trabecular pattern [Beaman et al 2004].
Histologic manifestations of lesions in the mandible and/or maxilla: non-neoplastic fibrotic lesions that contain numerous multinuclear giant cells and occasionally cysts. Increase in osteoid and newly formed bone matrix is observed in the periphery. Since cherubic phenotypes can be mimicked by other jaw tumors requiring different therapeutic strategies, a thoughtful consideration of histological analysis is required [Friedrich et al 2016].
Establishing the Diagnosis
The diagnosis of cherubism is established in a proband with typical clinical, radiographic, and histologic findings and/or a heterozygous pathogenic variant in SH3BP2 identified by molecular genetic testing (see Table 1).
Molecular genetic testing approaches can include a combination of gene-targeted testing (single-gene testing, multigene panel) and comprehensive genomic testing (exome sequencing, genome sequencing) depending on the phenotype.
Gene-targeted testing requires that the clinician determine which gene(s) are likely involved, whereas genomic testing does not. Because the phenotype of cherubism overlaps other conditions with jaw lesions, individuals with the distinctive findings described in Suggestive Findings are likely to be diagnosed using gene-targeted testing (see Option 1), whereas those with a phenotype indistinguishable from many other inherited disorders with jaw lesions or those in whom the diagnosis of cherubism has not been considered are more likely to be diagnosed using comprehensive genomic testing (see Option 2).
Option 1
When the phenotypic findings suggest the diagnosis of cherubism, molecular genetic testing approaches can include single-gene testing or use of a multigene panel.
- Single-gene testing. Sequence analysis of SH3BP2 detects small intragenic deletions/insertions and missense, nonsense, and splice site variants; typically, exon or whole-gene deletions/duplications are not detected. Perform sequence analysis first. If no pathogenic variant is found, gene-targeted deletion/duplication analysis could be considered, but since cherubism occurs through a gain-of-function mechanism and large intragenic deletion or duplication has not been reported, testing for intragenic deletions or duplication is unlikely to identify a disease-causing variant.Note: The majority of disease-associated variants have been reported in exon 9.
- A multigene panel that includes SH3BP2 and other genes of interest (see Differential Diagnosis) is most likely to identify the genetic cause of the condition at the most reasonable cost while limiting identification of variants of uncertain significance and pathogenic variants in genes that do not explain the underlying phenotype. Note: (1) The genes included in the panel and the diagnostic sensitivity of the testing used for each gene vary by laboratory and are likely to change over time. (2) Some multigene panels may include genes not associated with the condition discussed in this GeneReview. (3) In some laboratories, panel options may include a custom laboratory-designed panel and/or custom phenotype-focused exome analysis that includes genes specified by the clinician. (4) Methods used in a panel may include sequence analysis, deletion/duplication analysis, and/or other non-sequencing-based tests.For an introduction to multigene panels click here. More detailed information for clinicians ordering genetic tests can be found here.
Option 2
When the phenotype is indistinguishable from many other inherited disorders characterized by jaw lesions or the diagnosis of cherubism is not considered because an individual has atypical phenotypic features, comprehensive genomic testing (which does not require the clinician to determine which gene[s] are likely involved) is the best option. Exome sequencing is the most commonly used genomic testing method; genome sequencing is also possible.
For an introduction to comprehensive genomic testing click here. More detailed information for clinicians ordering genomic testing can be found here.
Table 1.
Molecular Genetic Testing Used in Cherubism
| Gene 1 | Test Method | Proportion of Probands with a Pathogenic Variant 2 Detectable by This Method |
|---|---|---|
| SH3BP2 | Sequence analysis 3 | ~80% 4 |
| Gene-targeted deletion/duplication analysis 5 | Unknown 6 | |
| Unknown 7 | NA |
- 1.
See Table A. Genes and Databases for chromosome locus and protein.
- 2.
See Molecular Genetics for information on allelic variants detected in this gene.
- 3.
Sequence analysis detects variants that are benign, likely benign, of uncertain significance, likely pathogenic, or pathogenic. Pathogenic variants may include small intragenic deletions/insertions and missense, nonsense, and splice site variants; typically, exon or whole-gene deletions/duplications are not detected. For issues to consider in interpretation of sequence analysis results, click here.
- 4.
Ueki et al [2001]
- 5.
Gene-targeted deletion/duplication analysis detects intragenic deletions or duplications. Methods used may include: quantitative PCR, long-range PCR, multiplex ligation-dependent probe amplification (MLPA), and a gene-targeted microarray designed to detect single-exon deletions or duplications.
- 6.
No data on detection rate of gene-targeted deletion/duplication analysis are available. Cherubism occurs through a gain-of-function mechanism; therefore, large intragenic deletions or duplications are unlikely to cause disease.
- 7.
Failure to identify a SH3BP2 pathogenic variant in 20% of affected individuals suggests possible genetic heterogeneity [Reichenberger et al 2012].
Clinical Characteristics
Clinical Description
Findings associated with cherubism range from clinically unrecognized features to severe mandibular and maxillary overgrowth with dental, orbital/ophthalmologic, respiratory, speech, and swallowing complications [Roginsky et al 2009]. Massive enlargement of the jaws is common and can also be associated with severe pain [Battaglia et al 2000, Timoşca et al 2000, Silva et al 2002, Gomes et al 2005, Wang et al 2006].
Wide variability in facial involvement within the same family was reported in the initial family described and in subsequent case series [Jones et al 1950, Li & Yu 2006, Preda et al 2010, Stoor et al 2017].
Onset and course. Individuals with cherubism have a normal appearance at birth. Usually, cherubism manifests in early childhood (age 2-7 years) and progresses until puberty, when it begins to stabilize and starts to regress. By age 30 years, the facial abnormalities associated with cherubism are not usually recognizable and residual deformity of the jaws is rare [Von Wowern 2000].
Presentation. The disease starts with rapid bone degradation, usually restricted to the mandibular and maxillary regions, and leads to multiple symmetric cystic changes. These cysts are filled with fibrous tissue mass that consists of stromal cells and osteoclast-like cells, resulting in the typical facial phenotype [Ozkan et al 2003].
- Submandibular and cervical lymph nodes are enlarged during the early stages of cherubism, and may present before the recognition of bony lesions.
- Mandibular cherubism is more frequent than maxillary [Schultze-Mosgau et al 2003].
- Aside from the facial anomalies described, cherubism is an isolated benign condition; the affected person has normal intellectual skills and is without other physical anomalies. The following rare exceptions have been reported:
- Lesions affecting the temporal bone, ribs, and humerus [Wayman 1978, Davis et al 1983, Fonseca et al 2004]
- A case of cherubism associated with spondyloarthropathy [Ling et al 2015]
Pathology of lesions. NFATc1 immunohistochemistry on lesional tissue may be helpful in defining a more aggressive form of the disease [Kadlub et al 2016]. The fibroosseous lesions in cherubism are limited to the mandible and maxilla, but the bone inflammatory findings are similar to other autoinflammatory syndromes with bone involvement, such as chronic recurrent multifocal osteomyelitis (CRMO) and deficiency of interleukin-1 receptor antagonist [Wipff et al 2011, Morbach et al 2013, Kadlub et al 2015, Bader-Meunier et al 2018]. One main difference in the pathology of cherubism, when compared to CRMO, is the degree of osteolysis, which is significantly more profound in cherubism [Wipff et al 2011].
Complications of cherubism may include the following:
- Jaw. Severe malformation of the jaw may impede physical functions such as chewing and swallowing, and may affect the social and psychological well-being of the individual, therefore forcing a decision on surgery [Pontes et al 2007, Papadaki et al 2012, Wright 2017]. See Treatment of Manifestations.
- Recurrence of the jaw lesions is possible after surgery.
- Although the cherubic appearance of patients is expected to be reduced due to involution of the bubble-like distensions of the jaws in early adulthood, this may not be the case for all [Redfors et al 2013].
- The facial disfigurement in cherubism can affect an individual's feelings of self-worth and be the source of bullying. However, a recent Scandinavian study reported that persons with cherubism were psychosocially well adapted and enjoyed a good quality of life [Prescott et al 2013].
- Difficulties with pronunciation have not been reported as a significant problem [Prescott et al 2013].
- Pain. An ache in the mouth and discomfort when eating food are frequently reported [Prescott et al 2013].
- Dental. In most affected persons, teeth are displaced, unerupted, unformed, or absent, or may appear to be floating in cystlike spaces. Malocclusion, premature exfoliation of deciduous teeth, and root resorption have also been reported [Kozakiewicz et al 2001, Stoor et al 2017].
- Respiratory. Respiratory problems can include obstructive sleep apnea and upper airway obstruction caused by backward displacement of the tongue [Battaglia et al 2000, Ladhani et al 2003, Khirani et al 2013].
- Orbital and ophthalmologic. Ophthalmic manifestations of cherubism vary greatly depending on the level of involvement of the maxilla [Mirmohammadsadeghi et al 2015, Yoo et al 2015]. In rare instances, enlargement of the maxilla and penetration of the stromal mass into the orbital floor can cause lower lid retraction, proptosis, strabismus, diplopia, globe displacement, and/or visual loss as a result of optic atrophy, retinal vein occlusion, and macular folds with scarring [Carroll & Sullivan 2001, Font et al 2003]. Prior reports of patients with maxillary involvement leading to orbital mass effect have varied in their ophthalmic sequelae and age of occurrence, with occurrence ranging from age seven to 27 years [Colombo et al 2001, Ahmadi et al 2003]
Genotype-Phenotype Correlations
No genotype/phenotype correlations have been described for cherubism.
Penetrance
Penetrance has not been systematically studied in cherubism. A previous report of reduced penetrance in females compared to males [Anderson &McClendon 1962] was subsequently shown to have bias in terms of how unaffected status was ascribed to the adult females [Reichenberger et al 2012].
Nomenclature
Cherubism was first described as "familial multilocular cystic disease of the jaws" by Jones [1933]; however, shortly thereafter he renamed the condition "cherubism" because of the resemblance of affected individuals to the cherubs in Renaissance art.
Prevalence
Prevalence is unknown. Approximately 300 cases have been reported in the medical literature. Variability of the cherubism phenotype may result in underdiagnosis of the condition in children, and as bone remodeling occurs into adulthood, there may be no evidence of previous disease on imaging in adults despite the presence of a SH3BP2 pathogenic variant.
Genetically Related (Allelic) Disorders
No other phenotypes are known to be associated with pathogenic variants in SH3BP2.
Differential Diagnosis
Table 2.
Disorders to Consider in the Differential Diagnosis of Cherubism
| Disorder | Gene(s) | MOI | Clinical Features | |
|---|---|---|---|---|
| Overlapping | Distinguishing | |||
RASopathies 1 including:
| BRAF MAP2K1 NF1 PTPN11 SOS1 KRAS LZTR1 MAP2K2 NRAS RAF1 RASA2 RRAS RIT1 SOS2 | AD | Giant-cell lesions of bones & soft tissues frequently found in jaws | RASopathies:
|
| Central giant-cell granuloma 2 | Unknown 3 | Unknown |
| Lesions in:
|
| Fibrous Dysplasia / McCune-Albright Syndrome (FD/MAS) | GNAS1 | See footnote 4 | Craniofacial form of FD/MAS may show clinical & radiologic overlap w/cherubism & thus be difficult to differentiate. 5 | Fibrous dysplasia:
|
| Hyperparathyroidism-jaw tumor (HPT-JT) syndrome (see CDC73-Related Disorders) | CDC73 | AD |
| HPT-JT: hyperparathyroidism |
| Familial isolated hyperparathyroidism (OMIM PS145000) | CDC73 MEN1 6 CASR GCM2 | AD AR |
| Hyperparathyroidism: ↑ serum concentrations of calcium, parathyroid hormone, & alkaline phosphatase 6 |
| Ramon syndrome (OMIM 266270) | Unknown | Unknown | Gingival fibromatosis | Ramon syndrome:
|
AD = autosomal dominant; AR = autosomal recessive; MOI = mode of inheritance; XL = X-linked
- 1.
RASopathies refers to disorders of the RAS-MAPK pathway.
- 2.
De Lange & Van den Akker [2005]
- 3.
A somatic pathogenic variant in SH3BP2 has been identified in one individual with central giant-cell granuloma [Carvalho et al 2009].
- 4.
Fibrous dysplasia / McCune-Albright syndrome, a sporadically occurring disorder, is caused by an early embryonic postzygotic somatic activating (gain-of-function) pathogenic variant in GNAS (encoding the cAMP pathway-associated G-protein, Gsα).
- 5.
Zohar et al [1989]
- 6.
Familial isolated hyperparathyroidism (FIHP) is characterized by parathyroid adenoma or hyperplasia without other associated endocrinopathies. Heterozygous MEN1 germline pathogenic variants have been reported in 20% [Miedlich et al 2001, Villablanca et al 2002] to 57% [Pannett et al 2003] of families with FIHP.
- 7.
Lessa et al [2005]
Cherubism has also been reported in (likely coincidental) association with a single case of coronal and sagittal craniosynostosis [Stiller et al 2000].
Management
Evaluations Following Initial Diagnosis
To establish the extent of disease in an individual diagnosed with cherubism, the following evaluations are recommended if they have not already been completed:
- Radiologic assessment to determine facial bone involvement. Computed tomography (CT utilizing the "as low as reasonably achievable" principle to minimize radiation) is currently the imaging modality most suitable to study bone lesions of the maxillofacial complex, enabling a precise evaluation of the components of the lesion [Pinheiro et al 2013]. However, evaluation of lesions encroaching on neurovascular or ocular structures may require a multimodality approach, which includes MRI [Cordeiro et al 2016, Purohit et al 2016]. The MRI findings of individual cherubism lesions are not specific to lesions caused by mutations in SH3BP2 [Beaman et al 2004, Jain & Sharma 2006].
- Evaluation in a craniofacial clinic including oral and maxillofacial surgery, plastic surgery, dentistry/orthodontics, otolaryngology, and child psychology or social work
- Orthodontic assessment to align tilted teeth is usually required after osseous growth is completed, but may be required concomitantly with fixed orthodontic appliances.
- Ophthalmologic examination
- Assessment of respiratory status including determining the presence or absence of mouth breathing, snoring, chronic nasal infection, and obstructive sleep apnea. An overnight polysomnogram should be considered if concerns regarding sleep-disordered breathing are present.
- Assessment by a speech therapist to include a swallowing assessment to determine if there is backward displacement of the tongue or obliteration of the nasal airway
- Consultation with a clinical geneticist and/or genetic counselor
Treatment of Manifestations
Treatment protocols for the complications of cherubism are not well established and are evolving due to recent advances in understanding the autoinflammatory nature of this bone disease. Given that cherubism is considered to be a self-limited condition that improves over time, treatment should be tailored to the individual's presentation and the evolution of the disease. Depending on the severity, surgery may be needed for functional and esthetic concerns.
- Children with cherubism should be referred to a craniofacial clinic with pediatric experience for ongoing management. A craniofacial clinic associated with a major pediatric medical center usually includes a surgical team, dentist, orthodontic specialist, ophthalmologist, and child psychologist or social worker.
- Surgical interventions include curettage with or without bone grafting [Kozakiewicz et al 2001, Roginsky et al 2009]. Liposuction has also been used successfully to re-contour the jaws. Surgical interventions are likely to occur between ages five and 15 years in individuals with disfiguring enlargement of jaws or locally aggressive lesions associated with complications such as impaired swallowing, respiratory issues, nasal airway obstruction, or tongue displacement.Surgical therapy needs to be individually tailored and not create unrealistic expectations since recurrence of the lesions is possible and surgery may not halt disease progression [Friedrich et al 2016].
- Orthodontic treatment is commonly required as the jaw distortion leads to permanent dental abnormalities including a malocclusive bite, premature loss of deciduous teeth, and widely spaced, misplaced, unerupted, or absent permanent teeth.
- Ophthalmologic treatment is necessary in rare individuals in whom orbital manifestations such as lower lid retraction, proptosis, diplopia, globe displacement, and visual loss caused by optic atrophy are present.
- Speech and language therapy may be necessary in rare cases where physical obstruction to the production of speech or swallowing is present.
Surveillance
Surveillance may reduce the risk for secondary complications such as visual impairment, upper airway obstruction, obstructive sleep apnea, and tooth displacement. Long-term follow up including clinical, radiographic, dental, orthodontic, and ophthalmologic evaluations is indicated [Silva et al 2007]. Expert-authored guidelines recommend annual reviews while new cysts continue to form or established cysts enlarge; after the disorder becomes quiescent, follow up every two to five years is recommended [Papadaki et al 2012].
Evaluation of Relatives at Risk
It is appropriate to evaluate apparently asymptomatic at-risk relatives in order to identify as early as possible those who would benefit from surveillance and early intervention. Evaluations can include:
- Molecular genetic testing if the pathogenic variant in the family is known.
- Clinical and radiographic evaluations if the pathogenic variant in the family is not known.
See Genetic Counseling for issues related to testing of at-risk relatives for genetic counseling purposes.
Therapies Under Investigation
Search ClinicalTrials.gov in the US and www.ClinicalTrialsRegister.eu in Europe for information on clinical studies for a wide range of diseases and conditions. Note: There may not be clinical trials for this disorder.