Neurodevelopmental Disorder With Or Without Anomalies Of The Brain, Eye, Or Heart
A number sign (#) is used with this entry because of evidence that this neurodevelopmental disorder with or without anomalies of the brain, eye, or heart (NEDBEH) is caused by heterozygous mutation in the RERE gene (605226) on chromosome 1p36.
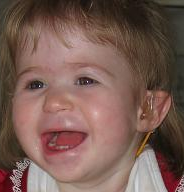
DescriptionNeurodevelopmental disorder with or without anomalies of the brain, eye, or heart is an autosomal dominant syndrome characterized by onset in infancy of developmental delay, intellectual disability, and behavioral disorders, such as autism spectrum disorders. About half of patients have additional abnormalities, most commonly involving the eye, heart, and genitourinary system. The phenotype is reminiscent of that observed in patients with 1p36 deletion syndrome (607872); RERE is located in the proximal 1p36 critical region (summary by Fregeau et al., 2016).
Clinical FeaturesFregeau et al. (2016) reported 10 unrelated children with a neurodevelopmental disorder characterized by delayed psychomotor development, intellectual disability, and autistic spectrum disorder, variably associated with additional congenital abnormalities. Two of the patients were previously reported (Krumm et al., 2015; Bosch et al., 2016). Features included intrauterine or postnatal growth retardation, feeding difficulties, hypotonia, and behavioral problems. Four patients had variable ophthalmologic abnormalities, including microphthalmia, iris anomalies, coloboma, Peter anomaly, optic atrophy, cortical visual impairment, strabismus, and blepharophimosis. Four patients had congenital heart defects, most commonly ventricular septal defect, and 5 patients had genitourinary defects, including vesicoureteral reflux, cryptorchidism, and hypospadias. Dysmorphic features, seen only in a few individuals, included frontal bossing, low-set, posteriorly rotated ears, epicanthal folds, anteverted nares, micrognathia, and broad eyebrows. Brain imaging in most patients showed ventriculomegaly, thin corpus callosum, small cerebellar vermis, and decreased white matter. Fregeau et al. (2016) found that the majority of features identified in the 10 patients overlapped with those observed in 31 individuals with 1p36 deletions involving RERE.
Molecular GeneticsFregeau et al. (2016) reported 10 unrelated patients with NEDBEH who carried a heterozygous mutation in the RERE gene (see, e.g., 605226.0001-605226.0004). The mutations, which were found by exome sequencing and confirmed by Sanger sequencing, occurred de novo in all cases in which parental DNA was available. The mutations clustered throughout the gene and included both missense and truncating mutations; functional studies of the variants and studies of patient cells were not performed. However, Fregeau et al. (2016) noted that mice with Rere mutations showed a similar phenotype (see ANIMAL MODEL). At least 3 patients (patients 2, 4, and 7) had variants in other genes that may or may not have contributed to the phenotype. Fregeau et al. (2016) suggested that haploinsufficiency of RERE may be sufficient to cause many of the phenotypes associated with proximal 1p36 deletions. One of the patients (patient 10) had previously been reported by Krumm et al. (2015).
Animal ModelKim et al. (2013) noted that mice homozygous for a null Rere allele died between E9.5 and E11.5 from failure of cardiac looping and subsequent cardiac failure. These embryos also had defects in somitogenesis, fusion of the telencephalic vesicles, defects of the optic vesicles and failure of anterior neural tube closure, and were given the name 'openmind' (om). Studies of these mice indicated that loss of Rere interfered with retinoic acid signaling and embryonic development. Kim et al. (2013) generated an allelic series of Rere-deficient mice using the 'om' allele and a hypomorphic Rere allele, termed 'eyes3' because it resulted in autosomal recessive microphthalmia. Mice compound heterozygous for both mutations had a high level of perinatal mortality, postnatal growth deficiency, brain hypoplasia, decreased numbers of hippocampal neurons, hearing loss, cardiovascular malformations, spontaneous development of cardiac fibrosis in adulthood, and renal agenesis. These findings suggested that Rere plays a critical role in the development and function of multiple organs including the eye, brain, inner ear, heart, and kidney. Kim et al. (2013) suggested that haploinsufficiency of RERE may contribute to the development of many of the phenotypes seen in human patients with 1p36 deletions. In a follow-up report, Fregeau et al. (2016) observed that om/eye3 compound heterozygous mice also had ventriculomegaly and incomplete closure of the optic fissure, suggestive of coloboma.
Kim and Scott (2014) specifically examined cerebellar development in the compound heterozygous Rere hypomorphic mice originally studied by Kim et al. (2013). Mutant mice showed pre- and postnatal delayed development of the principal fissures in the cerebellum, which was associated with decreased proliferative activity of granule cell precursors and delayed maturation and migration of Purkinje cells. These abnormalities were associated with a decrease in the expression of SHH (600725), which is secreted from Purkinje cells and is required for normal proliferation.