Coronavirus Disease 2019

Coronavirus disease 2019 (COVID-19) is a contagious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The first case was identified in Wuhan, China, in December 2019. It has since spread worldwide, leading to an ongoing pandemic.
Symptoms of COVID-19 are variable, but often include fever, cough, fatigue, breathing difficulties, and loss of smell and taste. Symptoms begin one to fourteen days after exposure to the virus. Around one in five infected individuals do not develop any symptoms. While most people have mild symptoms, some people develop acute respiratory distress syndrome (ARDS). ARDS can be precipitated by cytokine storms, multi-organ failure, septic shock, and blood clots. Longer-term damage to organs (in particular, the lungs and heart) has been observed. There is concern about a significant number of patients who have recovered from the acute phase of the disease but continue to experience a range of effects—known as long COVID—for months afterwards. These effects include severe fatigue, memory loss and other cognitive issues, low-grade fever, muscle weakness, and breathlessness.
The virus that causes COVID-19 spreads mainly when an infected person is in close contact with another person. Small droplets and aerosols containing the virus can spread from an infected person's nose and mouth as they breathe, cough, sneeze, sing, or speak. Other people are infected if the virus gets into their mouth, nose or eyes. The virus may also spread via contaminated surfaces, although this is not thought to be the main route of transmission. The exact route of transmission is rarely proven conclusively, but infection mainly happens when people are near each other for long enough. It can spread as early as two days before infected persons show symptoms, and from individuals who never experience symptoms. People remain infectious for up to ten days in moderate cases, and two weeks in severe cases. Various testing methods have been developed to diagnose the disease. The standard diagnosis method is by real-time reverse transcription polymerase chain reaction (rRT-PCR) from a nasopharyngeal swab.
Preventive measures include physical or social distancing, quarantining, ventilation of indoor spaces, covering coughs and sneezes, hand washing, and keeping unwashed hands away from the face. The use of face masks or coverings has been recommended in public settings to minimise the risk of transmissions. Several vaccines have been developed and various countries have initiated mass vaccination campaigns.
Although work is underway to develop drugs that inhibit the virus, the primary treatment is currently symptomatic. Management involves the treatment of symptoms, supportive care, isolation, and experimental measures.
Signs and symptoms

Symptoms of COVID-19 are variable, ranging from mild symptoms to severe illness. Common symptoms include headache, loss of smell and taste, nasal congestion and rhinorrhea, cough, muscle pain, sore throat, fever and breathing difficulties. People with the same infection may have different symptoms, and their symptoms may change over time. In people without prior ears, nose, and throat disorders, loss of taste combined with loss of smell is associated with COVID-19 with a specificity of 95%.
Most people (81%) develop mild to moderate symptoms (up to mild pneumonia), while 14% develop severe symptoms (dyspnea, hypoxia, or more than 50% lung involvement on imaging) and 5% of patients suffer critical symptoms (respiratory failure, shock, or multiorgan dysfunction). Around one in five people are infected with the virus but do not develop noticeable symptoms at any point in time. A June 2020 review asserted that asymptomatic infections might be as high as 40 to 45 percent with the ability to transmit the virus for a period that extends beyond two weeks. These asymptomatic carriers tend not to get tested, and they can spread the disease. Other infected people will develop symptoms later (called pre-symptomatic) or have very mild symptoms, and can also spread the virus.
As is common with infections, there is a delay, known as the incubation period, between the moment a person first becomes infected and the appearance of the first symptoms. The median incubation period for COVID-19 is four to five days. Most symptomatic people experience symptoms within two to seven days after exposure, and almost all symptomatic people will experience one or more symptoms before day twelve.Cause
COVID-19 is caused by infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus strain.
Transmission
COVID-19 spreads from person to person mainly through the respiratory route after an infected person coughs, sneezes, sings, talks or breathes. A new infection occurs when virus-containing particles exhaled by an infected person, either respiratory droplets or aerosols, get into the mouth, nose, or eyes of other people who are in close contact with the infected person. During human-to-human transmission, an average 1000 infectious SARS-CoV-2 virions are thought to initiate a new infection.
The closer people interact, and the longer they interact, the more likely they are to transmit COVID-19. Closer distances can involve larger droplets (which fall to the ground) and aerosols, whereas longer distances only involve aerosols. The larger droplets may also evaporate into the aerosols (known as droplet nuclei). The relative importance of the larger droplets and the aerosols is not clear as of November 2020, however the virus is not known to transmit between rooms over long distances such as through air ducts. Airborne transmission is able to particularly occur indoors, in high risk locations, such as in restaurants, choirs, gyms, nightclubs, offices, and religious venues, often when they are crowded or less ventilated. It also occurs in healthcare settings, often when aerosol-generating medical procedures are performed on COVID-19 patients.
Social distancing and the wearing of cloth face masks, surgical masks, respirators, or other face coverings are controls for droplet transmission. Transmission may be decreased indoors with well maintained heating and ventilation systems to maintain good air circulation and increase the use of outdoor air.
The number of people generally infected by one infected person varies; as of September 2020 it was estimated that one infected person will, on average, infect between two and three other people. This is more infectious than influenza, but less so than measles. It often spreads in clusters, where infections can be traced back to an index case or geographical location. There is a major role of "super-spreading events", where many people are infected by one person.Virology

Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel severe acute respiratory syndrome coronavirus. It was first isolated from three people with pneumonia connected to the cluster of acute respiratory illness cases in Wuhan. All features of the novel SARS-CoV-2 virus occur in related coronaviruses in nature.
Outside the human body, the virus is destroyed by household soap, which bursts its protective bubble.
SARS-CoV-2 is closely related to the original SARS-CoV. It is thought to have an animal (zoonotic) origin. Genetic analysis has revealed that the coronavirus genetically clusters with the genus Betacoronavirus, in subgenus Sarbecovirus (lineage B) together with two bat-derived strains. It is 96% identical at the whole genome level to other bat coronavirus samples (BatCov RaTG13). The structural proteins of SARS-CoV-2 include membrane glycoprotein (M), envelope protein (E), nucleocapsid protein (N), and the spike protein (S). The M protein of SARS-CoV-2 is 98.6% similar to the M protein of bat SARS-CoV, maintains 98.2% homology with pangolin SARS-CoV, and has 90% homology with the M protein of SARS-CoV; whereas, the similarity is only 38% with the M protein of MERS-CoV. In silico analyses showed that the M protein of SARS-CoV-2 has a triple helix bundle, forms a single 3-transmembrane domain, and is homologous to the prokaryotic sugar transport protein SemiSWEET.
The many thousands SARS-CoV-2 variants are grouped into clades. Several different clade nomenclatures have been proposed. Nextstrain divides the variants into five clades (19A, 19B, 20A, 20B, and 20C), while GISAID divdes them into seven (L, O, V, S, G, GH, and GR).
Several notable variants of SARS-CoV-2 emerged in the fall of 2020. Cluster 5 emerged among minks and mink farmers in Denmark. After strict quarantines and a mink euthanasia campaign, it is believed to have been eradicated. The Variant of Concern 202012/01 (VOC 202012/01) is believed to have emerged in the United Kingdom in September. The 501Y.V2 Variant, which has the same N501Y mutation, arose independently in South Africa.
Pathophysiology
COVID-19 can affect the upper respiratory tract (sinuses, nose, and throat) and the lower respiratory tract (windpipe and lungs). The lungs are the organs most affected by COVID-19 because the virus accesses host cells via the enzyme angiotensin-converting enzyme 2 (ACE2), which is most abundant in type II alveolar cells of the lungs. The virus uses a special surface glycoprotein called a "spike" (peplomer) to connect to ACE2 and enter the host cell. The density of ACE2 in each tissue correlates with the severity of the disease in that tissue and some have suggested decreasing ACE2 activity might be protective, though another view is that increasing ACE2 using angiotensin II receptor blocker medications could be protective. As the alveolar disease progresses, respiratory failure might develop and death may follow.
Whether SARS-CoV-2 is able to invade the nervous system remains unknown. The virus is not detected in the CNS of the majority of COVID-19 patients with neurological issues. However, SARS-CoV-2 has been detected at low levels in the brains of patients who died from COVID-19, but these results need to be confirmed. SARS-CoV-2 may cause respiratory failure through affecting the brain stem as other coronaviruses have been found to invade the CNS. While virus has been detected in cerebrospinal fluid of autopsies, the exact mechanism by which it invades the CNS remains unclear and may first involve invasion of peripheral nerves given the low levels of ACE2 in the brain. The virus may also enter the bloodstream from the lungs and cross the blood-brain barrier to gain access to the CNS, possibly within an infected white blood cell by a "Trojan horse" mechanism.
The virus also affects gastrointestinal organs as ACE2 is abundantly expressed in the glandular cells of gastric, duodenal and rectal epithelium as well as endothelial cells and enterocytes of the small intestine.
The virus can cause acute myocardial injury and chronic damage to the cardiovascular system. An acute cardiac injury was found in 12% of infected people admitted to the hospital in Wuhan, China, and is more frequent in severe disease. Rates of cardiovascular symptoms are high, owing to the systemic inflammatory response and immune system disorders during disease progression, but acute myocardial injuries may also be related to ACE2 receptors in the heart. ACE2 receptors are highly expressed in the heart and are involved in heart function. A high incidence of thrombosis and venous thromboembolism have been found in intensive care unit (ICU)-transferred patients with COVID-19 infections, and may be related to poor prognosis. Blood vessel dysfunction and clot formation (as suggested by high D-dimer levels) are thought to play a significant role in mortality, incidences of clots leading to pulmonary embolisms, and ischaemic events within the brain have been noted as complications leading to death in patients infected with SARS-CoV-2. Infection appears to set off a chain of vasoconstrictive responses within the body, constriction of blood vessels within the pulmonary circulation has also been posited as a mechanism in which oxygenation decreases alongside the presentation of viral pneumonia.
Another common cause of death is complications related to the kidneys. Early reports show that up to 30% of hospitalized patients both in China and in New York have experienced some injury to their kidneys, including some persons with no previous kidney problems.
Autopsies of people who died of COVID-19 have found diffuse alveolar damage (DAD), and lymphocyte-containing inflammatory infiltrates within the lung.
Immunopathology
Although SARS-CoV-2 has a tropism for ACE2-expressing epithelial cells of the respiratory tract, patients with severe COVID-19 have symptoms of systemic hyperinflammation. Clinical laboratory findings of elevated IL-2, IL-7, IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-γ inducible protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), Macrophage inflammatory protein 1-α (MIP-1α), and tumour necrosis factor-α (TNF-α) indicative of cytokine release syndrome (CRS) suggest an underlying immunopathology.
Additionally, people with COVID-19 and acute respiratory distress syndrome (ARDS) have classical serum biomarkers of CRS, including elevated C-reactive protein (CRP), lactate dehydrogenase (LDH), D-dimer, and ferritin.
Systemic inflammation results in vasodilation, allowing inflammatory lymphocytic and monocytic infiltration of the lung and the heart. In particular, pathogenic GM-CSF-secreting T-cells were shown to correlate with the recruitment of inflammatory IL-6-secreting monocytes and severe lung pathology in COVID-19 patients. Lymphocytic infiltrates have also been reported at autopsy.
Diagnosis


COVID-19 can provisionally be diagnosed on the basis of symptoms and confirmed using reverse transcription polymerase chain reaction (RT-PCR) testing of infected secretions. Along with laboratory testing, chest CT scans may be helpful to diagnose COVID-19 in individuals with a high clinical suspicion of infection. Detection of prior infection is possible with serological tests, which detect antibodies produced by the body in response to infection.
Viral testing
The standard method of testing for presence of SARS-CoV-2 is real-time reverse transcription polymerase chain reaction (rRT-PCR), which detects the presence of viral RNA fragments. As this test detects RNA but not infectious virus, its "ability to determine duration of infectivity of patients is limited." The test is typically done on respiratory samples obtained by a nasopharyngeal swab; however, a nasal swab or sputum sample may also be used. Results are generally available within a few hours to two days. Blood tests can be used, but these require two blood samples taken two weeks apart, and the results have little immediate value. The WHO has published several testing protocols for the disease.
A number of laboratories and companies have developed serological tests, which detect antibodies produced by the body in response to infection. Several have been evaluated by Public Health England and approved for use in the UK.
On 22 June 2020, UK health secretary Matt Hancock announced the country would conduct a new "spit test" for COVID-19 on 14,000 key workers and their families in Southampton, having them spit in a pot, which was collected by Southampton University, with results expected within 48 hours. Hancock said the test was easier than using swabs and could enable people to conduct it at home.
The University of Oxford's CEBM has pointed to mounting evidence that "a good proportion of 'new' mild cases and people re-testing positives after quarantine or discharge from hospital are not infectious, but are simply clearing harmless virus particles which their immune system has efficiently dealt with" and have called for "an international effort to standardize and periodically calibrate testing" On 7 September, the UK government issued "guidance for procedures to be implemented in laboratories to provide assurance of positive SARS-CoV-2 RNA results during periods of low prevalence, when there is a reduction in the predictive value of positive test results."
Chinese scientists were able to isolate a strain of the coronavirus and publish the genetic sequence so laboratories across the world could independently develop polymerase chain reaction (PCR) tests to detect infection by the virus. As of 4 April 2020[update], antibody tests (which may detect active infections and whether a person had been infected in the past) were in development, but not yet widely used. Antibody tests may be most accurate 2–3 weeks after a person's symptoms start. The Chinese experience with testing has shown the accuracy is only 60 to 70%. The US Food and Drug Administration (FDA) approved the first point-of-care test on 21 March 2020 for use at the end of that month. The absence or presence of COVID-19 signs and symptoms alone is not reliable enough for an accurate diagnosis. Different clinical scores were created based on symptoms, laboratory parameters and imaging to determine patients with probable SARS-CoV-2 infection or more severe stages of COVID-19.
A study asked hospitalised COVID-19 patients to cough into a sterile container, thus producing a saliva sample, and detected the virus in eleven of twelve patients using RT-PCR. This technique has the potential of being quicker than a swab and involving less risk to health care workers (collection at home or in the car).
In November 2020, research showed that breath analysis could make the "rapid identification" in seconds for coronavirus possible.
Imaging
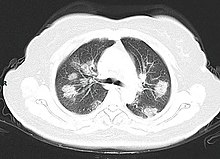
Chest CT scans may be helpful to diagnose COVID-19 in individuals with a high clinical suspicion of infection but are not recommended for routine screening. Bilateral multilobar ground-glass opacities with a peripheral, asymmetric, and posterior distribution are common in early infection. Subpleural dominance, crazy paving (lobular septal thickening with variable alveolar filling), and consolidation may appear as the disease progresses. Characteristic imaging features on chest radiographs and computed tomography (CT) of people who are symptomatic include asymmetric peripheral ground-glass opacities without pleural effusions.
Many groups have created COVID-19 datasets that include imagery such as the Italian Radiological Society which has compiled an international online database of imaging findings for confirmed cases. Due to overlap with other infections such as adenovirus, imaging without confirmation by rRT-PCR is of limited specificity in identifying COVID-19. A large study in China compared chest CT results to PCR and demonstrated that though imaging is less specific for the infection, it is faster and more sensitive.

CT scan of rapid progression stage of COVID-19.

Chest X-ray showing COVID-19 pneumonia.
Coding
In late 2019, the WHO assigned emergency ICD-10 disease codes U07.1 for deaths from lab-confirmed SARS-CoV-2 infection and U07.2 for deaths from clinically or epidemiologically diagnosed COVID-19 without lab-confirmed SARS-CoV-2 infection.
Pathology
The main pathological findings at autopsy are:
- Macroscopy: pericarditis, lung consolidation and pulmonary oedema
- Lung findings:
- minor serous exudation, minor fibrin exudation
- pulmonary oedema, pneumocyte hyperplasia, large atypical pneumocytes, interstitial inflammation with lymphocytic infiltration and multinucleated giant cell formation
- diffuse alveolar damage (DAD) with diffuse alveolar exudates. DAD is the cause of acute respiratory distress syndrome (ARDS) and severe hypoxemia.
- organisation of exudates in alveolar cavities and pulmonary interstitial fibrosis
- plasmocytosis in BAL
- Blood: disseminated intravascular coagulation (DIC); leukoerythroblastic reaction
- Liver: microvesicular steatosis
Prevention


Preventive measures to reduce the chances of infection include staying at home, wearing a mask in public, avoiding crowded places, keeping distance from others, ventilating indoor spaces, washing hands with soap and water often and for at least 20 seconds, practising good respiratory hygiene, and avoiding touching the eyes, nose, or mouth with unwashed hands. Those diagnosed with COVID-19 or who believe they may be infected are advised by the CDC to stay home except to get medical care, call ahead before visiting a healthcare provider, wear a face mask before entering the healthcare provider's office and when in any room or vehicle with another person, cover coughs and sneezes with a tissue, regularly wash hands with soap and water and avoid sharing personal household items.
The first COVID-19 vaccine was granted regulatory approval on 2 December by the UK medicines regulator MHRA. It was evaluated for emergency use authorization (EUA) status by the US FDA, and in several other countries. Initially, the US National Institutes of Health guidelines do not recommend any medication for prevention of COVID-19, before or after exposure to the SARS-CoV-2 virus, outside the setting of a clinical trial. Without a vaccine, other prophylactic measures, or effective treatments, a key part of managing COVID-19 is trying to decrease and delay the epidemic peak, known as "flattening the curve". This is done by slowing the infection rate to decrease the risk of health services being overwhelmed, allowing for better treatment of current cases, and delaying additional cases until effective treatments or a vaccine become available.
Vaccine
 28/COVID_Vaccine_%2850745583447%29.jpg/220px-COVID_Vaccine_%2850745583447%29.jpg" decoding="async" width="220" height="147" class="thumbimage" srcset="//upload.wikimedia.org/wikipedia/commons/thumb/2/28/COVID_Vaccine_%2850745583447%29.jpg/330px-COVID_Vaccine_%2850745583447%29.jpg 1.5x, //upload.wikimedia.org/wikipedia/commons/thumb/2/28/COVID_Vaccine_%2850745583447%29.jpg/440px-COVID_Vaccine_%2850745583447%29.jpg 2x" data-file-width="6000" data-file-height="4000">
28/COVID_Vaccine_%2850745583447%29.jpg/220px-COVID_Vaccine_%2850745583447%29.jpg" decoding="async" width="220" height="147" class="thumbimage" srcset="//upload.wikimedia.org/wikipedia/commons/thumb/2/28/COVID_Vaccine_%2850745583447%29.jpg/330px-COVID_Vaccine_%2850745583447%29.jpg 1.5x, //upload.wikimedia.org/wikipedia/commons/thumb/2/28/COVID_Vaccine_%2850745583447%29.jpg/440px-COVID_Vaccine_%2850745583447%29.jpg 2x" data-file-width="6000" data-file-height="4000"> 
A COVID‑19 vaccine is a vaccine intended to provide acquired immunity against COVID‑19. Prior to the COVID‑19 pandemic, work to develop a vaccine against the coronavirus diseases SARS and MERS had established knowledge about the structure and function of coronaviruses, which accelerated development during early 2020 of varied technology platforms for a COVID‑19 vaccine.
By mid-December 2020, 57 vaccine candidates were in clinical research, including 40 in Phase I–II trials and 17 in Phase II–III trials. In Phase III trials, several COVID‑19 vaccines demonstrated efficacy as high as 95% in preventing symptomatic COVID‑19 infections. As of January 2021, nine vaccines have been authorized by at least one national regulatory authority for public use: two RNA vaccines (the Pfizer-BioNTech vaccine and the Moderna vaccine), three conventional inactivated vaccines (BBIBP-CorV from Sinopharm, BBV152 from Bharat Biotech and CoronaVac from Sinovac), three viral vector vaccines (Gam-COVID-Vac from the Gamaleya Research Institute, the Oxford–AstraZeneca vaccine, and Sputnik V), and one peptide vaccine (EpiVacCorona).
Many countries have implemented phased distribution plans that prioritize those at highest risk of complications, such as the elderly, and those at high risk of exposure and transmission, such as healthcare workers. As of 14 January 2021, 32.64 million doses of COVID‑19 vaccine had been administered worldwide based on official reports from national health agencies. Pfizer, Moderna, and AstraZeneca predicted a manufacturing capacity of 5.3 billion doses in 2021, which could be used to vaccinate about 3 billion people (as the vaccines require two doses for a protective effect against COVID‑19). By December, more than 10 billion vaccine doses had been preordered by countries, with about half of the doses purchased by high-income countries comprising only 14% of the world's population.Social distancing
Social distancing (also known as physical distancing) includes infection control actions intended to slow the spread of the disease by minimising close contact between individuals. Methods include quarantines; travel restrictions; and the closing of schools, workplaces, stadiums, theatres, or shopping centres. Individuals may apply social distancing methods by staying at home, limiting travel, avoiding crowded areas, using no-contact greetings, and physically distancing themselves from others. Many governments are now mandating or recommending social distancing in regions affected by the outbreak. Non-cooperation with distancing measures in some areas has contributed to the further spread of the pandemic. Initial recommendations included maintaining a six-foot/two-meter distance from others outside the family unit. However, a case occurring in South Korea suggested that is inadequate, citing transmission despite a brief exposure (5 minutes) at 20 feet from the carrier in a restaurant. The maximum gathering size recommended by U.S. government bodies and health organizations was swiftly reduced from 250 people (if there were no known COVID-19 spread in a region) to 50 people, and later to 10. A Cochrane review found that early quarantine with other public health measures are effective in limiting the pandemic. The best manner of adopting and relaxing policies are uncertain, however, as local conditions vary.
Older adults and those with underlying medical conditions such as diabetes, heart disease, respiratory disease, hypertension, and compromised immune systems face increased risk of serious illness and complications and have been advised by the CDC to stay home as much as possible in areas of community outbreak.
In late March 2020, the WHO and other health bodies began to replace the use of the term "social distancing" with "physical distancing", to clarify that the aim is to reduce physical contact while maintaining social connections, either virtually or at a distance. The use of the term "social distancing" had led to implications that people should engage in total social isolation, rather than encouraging them to stay in contact through alternative means. Some authorities have issued sexual health guidelines for responding to the pandemic, which include recommendations to have sex only with someone with whom you live, and who does not have the virus or symptoms of the virus.
Outbreaks have occurred in prisons due to crowding and an inability to enforce adequate social distancing. In the United States, the prisoner population is aging and many of them are at high risk for poor outcomes from COVID-19 due to high rates of coexisting heart and lung disease, and poor access to high-quality healthcare.
Self-isolation
Self-isolation at home has been recommended for those diagnosed with COVID-19 and those who suspect they have been infected. Health agencies have issued detailed instructions for proper self-isolation.
Many governments have mandated or recommended self-quarantine for entire populations. The strongest self-quarantine instructions have been issued to those in high-risk groups. Those who may have been exposed to someone with COVID-19 and those who have recently travelled to a country or region with the widespread transmission have been advised to self-quarantine for 14 days from the time of last possible exposure.
Face masks and respiratory hygiene
The WHO and the US CDC recommend individuals wear non-medical face coverings in public settings where there is an increased risk of transmission and where social distancing measures are difficult to maintain. This recommendation is meant to reduce the spread of the disease by asymptomatic and pre-symptomatic individuals and is complementary to established preventive measures such as social distancing. Face coverings limit the volume and travel distance of expiratory droplets dispersed when talking, breathing, and coughing. Face coverings also filter out particles containing the virus from inhaled air, reducing the chance that the wearer will become infected. Many countries and local jurisdictions encourage or mandate the use of face masks or cloth face coverings by members of the public to limit the spread of the virus.
Masks are also strongly recommended for those who may have been infected and those taking care of someone who may have the disease. When not wearing a mask, the CDC recommends covering the mouth and nose with a tissue when coughing or sneezing and recommends using the inside of the elbow if no tissue is available. Proper hand hygiene after any cough or sneeze is encouraged. Healthcare professionals interacting directly with COVID-19 patients are advised to use respirators at least as protective as NIOSH-certified N95 or equivalent, in addition to other personal protective equipment.
Hand-washing and hygiene
When not wearing a mask, the CDC, the WHO, and the NHS recommend covering the mouth and nose with a tissue when coughing or sneezing and recommends using the inside of the elbow if no tissue is available. Proper hand hygiene after any cough or sneeze is encouraged. The WHO also recommends that individuals wash hands often with soap and water for at least 20 seconds, especially after going to the toilet or when hands are visibly dirty, before eating and after blowing one's nose. The CDC recommends using an alcohol-based hand sanitiser with at least 60% alcohol, but only when soap and water are not readily available. For areas where commercial hand sanitisers are not readily available, the WHO provides two formulations for local production. In these formulations, the antimicrobial activity arises from ethanol or isopropanol. Hydrogen peroxide is used to help eliminate bacterial spores in the alcohol; it is "not an active substance for hand antisepsis". Glycerol is added as a humectant.
Surface cleaning
Coronaviruses on surfaces die "within hours to days". Coronaviruses die faster when exposed to sunlight and warm temperatures.
Surfaces may be decontaminated with a number of solutions (within one minute of exposure to the disinfectant for a stainless steel surface), including 62–71 percent ethanol, 50–100 percent isopropanol, 0.1 percent sodium hypochlorite, 0.5 percent hydrogen peroxide, and 0.2–7.5 percent povidone-iodine. Other solutions, such as benzalkonium chloride and chlorhexidine gluconate, are less effective. Ultraviolet germicidal irradiation may also be used. The CDC recommends that if a COVID-19 case is suspected or confirmed at a facility such as an office or day care, all areas such as offices, bathrooms, common areas, shared electronic equipment like tablets, touch screens, keyboards, remote controls, and ATM machines used by the ill persons should be disinfected.
Ventilation and air filtration
The WHO recommends ventilation and air filtration in public spaces to help clear out infectious aerosols.
Healthy diet and lifestyle
The Harvard T.H. Chan School of Public Health recommends a healthy diet, being physically active, managing psychological stress, and getting enough sleep. Dark-skinned people are at particular risk of a vitamin D deficiency which can impair the immune system.
Treatment
There is no specific, effective treatment or cure for coronavirus disease 2019 (COVID-19), the disease caused by the SARS-CoV-2 virus. Thus, the cornerstone of management of COVID-19 is supportive care, which includes treatment to relieve symptoms, fluid therapy, oxygen support and prone positioning as needed, and medications or devices to support other affected vital organs.
Most cases of COVID-19 are mild. In these, supportive care includes medication such as paracetamol or NSAIDs to relieve symptoms (fever, body aches, cough), proper intake of fluids, rest, and nasal breathing. Good personal hygiene and a healthy diet are also recommended. The U.S. Centers for Disease Control and Prevention (CDC) recommend that those who suspect they are carrying the virus isolate themselves at home and wear a face mask.
People with more severe cases may need treatment in hospital. In those with low oxygen levels, use of the glucocorticoid dexamethasone is strongly recommended, as it can reduce the risk of death. Noninvasive ventilation and, ultimately, admission to an intensive care unit for mechanical ventilation may be required to support breathing. Extracorporeal membrane oxygenation (ECMO) has been used to address the issue of respiratory failure, but its benefits are still under consideration.
Several experimental treatments are being actively studied in clinical trials. Others were thought to be promising early in the pandemic, such as hydroxychloroquine and lopinavir/ritonavir, but later research found them to be ineffective or even harmful. Despite ongoing research, there is still not enough high-quality evidence to recommend so-called early treatment. Nevertheless, in the United States, two monoclonal antibody-based therapies are available for early use in cases thought to be at high risk of progression to severe disease. The antiviral remdesivir is available in the U.S., Canada, Australia, and several other countries, with varying restrictions; however, it is not recommended for people needing mechanical ventilation, and is discouraged altogether by the World Health Organization (WHO), due to limited evidence of its efficacy.



