Romano-Ward Syndrome
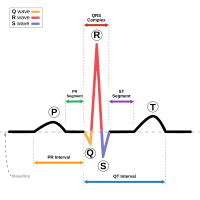
A form of familial long QT syndrome (LQTS) characterized by syncopal episodes and electrocardiographic abnormalities (QT prolongation, T-wave abnormalities and torsade de pointes (TdP) ventricular tachycardia).
Epidemiology
The prevalence of Romano-Ward syndrome (RWS) is estimated at 1/2,500.
Clinical description
Cardiac events occur from infancy through middle age but may manifest as early as the intrauterine stage with possibility of still birth. Most patients develop the symptoms during exercise or in response to stress or emotional disturbances and symptoms rarely occur at rest or during sleep. The syncopal episodes are due to TdP, a polymorphic ventricular tachycardia. TdP often degenerates to ventricular fibrillation and causes cardiac arrest or sudden death. In some patients, cardiac arrest may be the first manifestation of the disease. The electrocardiogram typically shows a prolongation of ventricular repolarization (QTc > 460 ms) and biphasic or notched T-waves in the precordial leads. Beat-to-beat alternation of the T wave (in polarity or amplitude) may be present at rest, but most commonly appears during emotional or physical stress and may precede TdP. The heart rate at rest or during exercise may be slower than normal.
Etiology
RWS may result from mutations in genes either encoding subunits of cardiac ion channels or proteins interacting with cardiac ion channels. To date pathogenic mutations have been identified in numerous genes, the most frequently observed causative genes are KCNQ1, KCNH2, SCN5A. The genes CALM1, CALM2 and TRDN have also undisputable evidence as LQTS-causative genes, while the remaining genes have moderate to limited evidence for LQTS causation.
Diagnostic methods
Diagnosis is based on typical electrocardiographic findings, clinical manifestations and family history. Molecular diagnosis should always be performed in patients with a clinically suspected diagnosis. It should also be performed on affected family members with normal/borderline QT intervals to identify those at risk of sudden death.
Differential diagnosis
Typical cases are so characteristic that they do not require differential diagnosis. For borderline cases, the following conditions should be considered: catecholaminergic polymorphic ventricular tachycardia (CPVT), Jervell and Lange-Nielsen syndrome and other forms of LQTS, as well as vasovagal syncope, ventricular tachycardia, drug-induced LQTS and epilepsy.
Antenatal diagnosis
Prenatal testing may be available for families in which the disease-causing mutation is known.
Genetic counseling
The pattern of inheritance is autosomal dominant and genetic counseling is recommended for affected families. There is a 50% risk of transmitting the pathogenic variant from an affected individual to their offspring. Autosomal recessive transmission has been described in very few cases of RWS, and in some with TRDN mutations only.
Management and treatment
Beta-adrenergic blockers represent the first choice therapy in symptomatic patients. Whenever syncopal episodes recur despite full-dose beta-blocking therapy, left cardiac sympathetic denervation (LCSD) should be considered and implemented whenever possible. Cardiac pacing is only rarely indicated (e.g. in infants or young children with 2:1 atrioventricular block). Implantable cardioverter defibrillators (ICDs) are always indicated after cardiac arrest, or when requested by the patient, and whenever syncope recurs despite beta-blockade and LCSD. Prophylactic use of beta blockers is indicated in asymptomatic children and adults under age 40 years with LQTS (LQT1, LQT2 or LQT3). LQTS and its variants are leading causes of sudden cardiac death in young, otherwise healthy, subjects and contribute significantly to sudden infant death syndrome (SIDS). As very effective therapies exist, early diagnosis (neonatal ECG screening) is of crucial importance and permits preventive management.
Prognosis
The prognosis is severe for infants with arrhythmic events in the first year of life and for those with mutations in the Calmodulin genes.