Telangiectasia, Hereditary Hemorrhagic, Type 1
A number sign (#) is used with this entry because hereditary hemorrhagic telangiectasia type 1 (HHT1) is caused by heterozygous mutation in the gene encoding endoglin (ENG; 131195) on chromosome 9q34.
DescriptionHereditary hemorrhagic telangiectasia (HHT) is an autosomal dominant vascular dysplasia leading to telangiectases and arteriovenous malformations of skin, mucosa, and viscera. Epistaxis and gastrointestinal bleeding are frequent complications of mucosal involvement. Visceral involvement includes that of the lung, liver, and brain. The most frequent form of hereditary hemorrhagic telangiectasia maps to the long arm of chromosome 9.
Genetic Heterogeneity of Hereditary Hemorrhagic Telangiectasia
See also HHT2 (600376), caused by mutation in the ALK1 gene (ACVRL1; 601284) on chromosome 12q13; HHT3 (601101), mapped to chromosome 5q31; HHT4 (610655), mapped to chromosome 7p14; and HHT5 (615506), caused by mutation in the GDF2 gene (605120) on chromosome 10q11.
See also juvenile polyposis/HHT syndrome (175050), caused by mutation in the SMAD4 gene (600993).
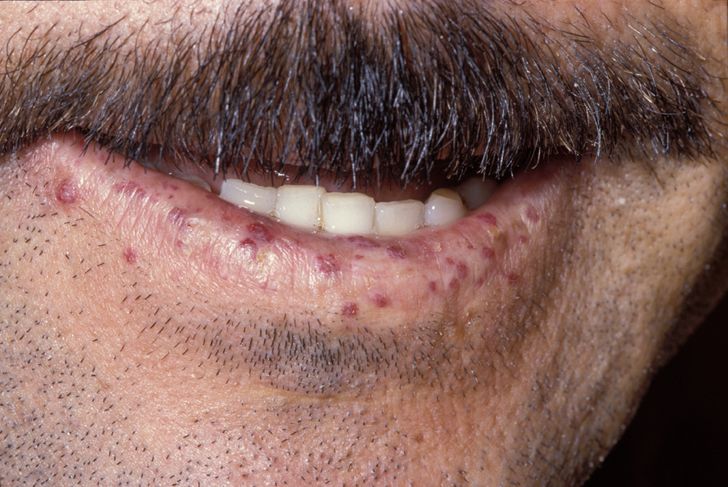
Clinical FeaturesHHT is highly penetrant; in a series of 384 patients, Plauchu et al. (1989) found at least 1 manifestation in 97%, while Porteous et al. (1992) found complete penetrance by 40 years of age in a series of 35 British families with 98 affected members. Sixty-two percent of these were clinically affected by age 16, with epistaxes being the presenting feature in 90% of cases. Aassar et al. (1991) found that the mean age of onset of epistaxis in HHT was 12 years, with more than 90% becoming manifest before 21 years. Blood loss from the nasal mucosa may become severe. Telangiectases also occur on the mucosal surface of the tongue (where bleeding may prove difficult to control), lips, face, conjunctiva, ears and fingers. Plauchu et al. (1989) noted facial involvement in 33% and lesions on the hands or wrists of 41% of their patients
Porteous et al. (1992) found significant gastrointestinal hemorrhage in 16% of patients, with half of these requiring transfusion. The preponderance of upper GI involvement may have reflected the reliance on upper GI endoscopy.
By angiographic methods, various types of visceral angiodysplasias have been demonstrated (Halpern et al., 1968). These include arterial aneurysm, arteriovenous communication including discrete arteriovenous fistula, conglomerate masses of angiectasia, phlebectasia, and angioma. Pulmonary arteriovenous malformations (PAVMs) are a significant cause of morbidity. Some are sufficiently large to cause heart failure leading to polycythaemia and clubbing. Paradoxical emboli may cause infarction or abscess formation in the brain and elsewhere. Vase et al. (1985) reported PAVMs in 20% of their series. Plauchu et al. (1989) found PAVMs in 4.6% with an age range of 1 to 78 years. In the study of Porteous et al. (1992) 13 (23%) of those who had undergone chest radiography had a visible PAVM; 4 suffered embolic complications, 3 cerebral abscesses, and one a stroke. In one 17-year-old, 50% of the circulating volume was passing through a single PAVM. Reyes-Mujica et al. (1988) described HHT in a 23-month-old girl who died of massive pulmonary hemorrhage. There were no skin lesions but vascular anomalies of varying severity were found in the tongue, esophagus, liver, kidney, central nervous system, ovaries, spleen, and lymph nodes. Before death, the child had 15 episodes of hemoptysis and 2 of epistaxis. The parents, by contrast, had no evidence of the disease but 1 grandfather had died after bleeding from the mouth following physical exertion. Dines et al. (1974) reviewed 63 cases of pulmonary arteriovenous fistula seen at the Mayo Clinic; HHT was recognized in 38 (60%).
Cirrhosis of the liver may occur; Plauchu et al. (1989) found liver involvement in 27 patients (8%); 17 of these had cirrhosis, which was the cause of death in 5. Michaeli et al. (1968) described a 47-year-old woman with HHT disease and hepatic portacaval shunts of sufficient magnitude to cause repeated episodes of encephalopathy. The liver was not scarred. Nikolopoulos et al. (1988) raised the question of familial tendency to hepatic involvement in HHT. They described 2 brothers with intrahepatic arteriovenous shunts of sufficient size to cause hyperdynamic circulation, leading to cirrhosis; the mother and 3 maternal uncles died of cirrhosis with rupture of esophageal varices. Members of the previous generation also had a history of hyperdynamic circulation. Selmaier et al. (1993) described the case of a 50-year-old woman with heart failure resulting from calcified hemangiomatosis of the liver with a high shunt volume. Saxena et al. (1998) reported the case of a 43-year-old woman who received a transplant for end-stage liver disease due to HHT and fibropolycystic liver disease. The liver showed extensive vascular malformations of arteries and veins, as well as telangiectasia and fibrosis. In addition, there were cystically dilated ducts containing inspissated bile and extensive von Meyenburg complexes. The case raised the question of a possible relationship between polycystic liver disease (174050) and HHT. (A von Meyenburg complex consists of clusters of small bile ducts occurring in polycystic livers, separate from the portal areas.)
In the liver, the vascular abnormalities of HHT are associated with marked fibrosis and/or cirrhosis. Weik and Greiner (1999) found hepatic manifestations of HHT in 4 women and 1 man (51 to 63 years of age) presenting initially with slight disturbances of liver function. In 3 patients, progressive liver insufficiency developed.
Garcia-Tsao et al. (2000) described the clinical findings and results of hemodynamic, angiographic, and imaging studies in 19 patients with HHT and symptomatic liver involvement. Ages ranged from 34 to 74 years in the 14 women and 5 men. All but 1 had a hyperdynamic circulation (cardiac index, 4.2 to 7.3 liters per minute per square meter of body-surface area). In 8 patients, the clinical findings were consistent with the presence of high-output heart failure. Manifestations of portal hypertension such as ascites or variceal bleeding were present in 6 patients. Manifestations of biliary disease, such as an elevated alkaline phosphatase level and abnormalities on bile duct imaging, were present in 5 patients. One of these patients died after an unsuccessful attempt at liver transplantation.
Cooke (1986) described renal arteriovenous malformations in a patient with episodic hematuria and renal colic due to clots.
Telangiectases may occur in the bladder, although Plauchu et al. (1989) found only 2 symptomatic patients among their 324 cases. Kurnik and Heymann (1989) described 3-vessel coronary artery ectasia without evidence of atherosclerosis in a 51-year-old man with classic HHT disease. This manifestation had not previously been described although ectasia of other vessels such as intraabdominal ones is well known. In a study of 20 patients with HHT, Brant et al. (1989) found conjunctival telangiectases in 7 and retinal vascular malformations in 2. Visual loss from the intraocular lesions is a rare complication. Bloody tears sometimes occur in patients with conjunctival telangiectases and bleeding from the eyes may also result from the backing up of blood in the lacrimal duct during epistaxis with packing of the nostrils.
Most of the neurologic morbidity is related to emboli but vascular malformations may occur; Guillen et al. (1991) found 1 individual, in a Mexican family with 15 affected members, who needed surgical treatment for a cerebral lesion, while 3 of the patients seen by Porteous et al. (1992) had symptomatic cerebral lesions. In the latter report, 46.3% of patients with no known CNS pathology described visual symptoms suggestive of migrainous aura in the absence of headache and nausea compared to 5.7% of controls. Steele et al. (1993) investigated migraine prevalence in 58 British adult HHT gene carriers without known neurologic deficits; 40 carriers of the gene for familial adenomatous polyposis (FAP; 175100) were used as controls. They found that 50% of the HHT carriers fulfill diagnostic criteria for migraine with aura, 4 times the disease control group and 10 times the estimated population prevalence. White had observed this symptom separately and noted that headaches improved in patients who had undergone balloon occlusion of PAVMs (White et al., 1988). This raises the possibility of vasoactive substances which would normally be removed in the pulmonary vascular bed reaching the central nervous system, though if this is the explanation it would suggest that almost half of gene carriers have pulmonary involvement. Another factor may be occult intracranial AVMs; 6 to 8% of HHT patients with PAVMs also have intracranial lesions (Roman et al., 1978).
Fulbright et al. (1998) reviewed brain magnetic resonance imaging (MRI) of 184 consecutive patients with HHT. Catheter angiography was performed in 17 patients in whom cerebrovascular malformations (CVMs) were detected on MRIs. They found 63 CVMs in 42 patients. Classic arteriovenous malformations (n = 10) had a conspicuous network of vessels with flow voids and enlarged adjacent pial vessels. Apparent venous malformations (n = 5) were best seen after administration of contrast material as a prominent vessel coursing through normal brain parenchyma. Indeterminate vascular malformations (n = 48) had a spectrum of appearances characterized by variable combinations of heterogeneous signal intensity, enhancement, or hemosiderin. Angiography in 17 patients revealed 47 CVMs. Forty-six were arteriovenous malformations (AVMs), including 25 CVMs not seen with MRI and 21 CVMs that by MR criteria included 8 AVMs and 13 indeterminate vascular malformations. Angiography confirmed 1 venous malformation seen with MRI but failed to detect 3 indeterminate lesions revealed by MRI. Thus, MRI revealed a CVM prevalence of 23% (42 of 184). Most CVMs (48 of 63) had an atypical appearance for vascular malformations on MR images. Angiographic correlation suggests that MRI underestimates the prevalence of CVMs and that the majority of indeterminate CVMs, despite their variable MRI appearance, are AVMs.
Kopel and Lage (1998) described a 37-year-old woman with HHT who developed a large pericardial effusion with cardiac tamponade. Pericardiocentesis yielded a large amount of hemorrhagic pericardial fluid. Because of recurrent cardiac tamponade, the patient underwent partial surgical pericardial excision. Histologic examination of the pericardium showed vascular dysplasia with signs of hemorrhage and inflammation.
Canzonieri et al. (2014) examined the gastrointestinal tract of consecutive HHT patients to assess distribution, number, size, and type of telangiectases in relation to genotype. Twenty-two patients (13 men; mean age 59 +/- 9 years) were analyzed, 7 with HHT1, 13 with HHT2 (600376), and 2 undefined. Gastrointestinal telangiectases were identified in 86% of HHT1 patients and in 77% of HHT2 patients.
Reviews
Guttmacher et al. (1995) reviewed all aspects of HHT. They emphasized that it is important for those affected to be aware of their diagnosis and its implications and to inform health care providers of their condition. Guttmacher et al. (1995) announced that educational materials for patients and providers are available from the HHT Foundation International, Inc.
Haitjema et al. (1996) provided a review. Marchuk et al. (1998) reported on a 1997 workshop on hereditary hemorrhagic telangiectasia.
Govani and Shovlin (2009) reviewed the molecular and genetic basis of hereditary hemorrhagic telangiectasia and discussed approaches for diagnosis and clinical management.
InheritanceHHT disease is inherited as an autosomal dominant trait. Snyder and Doan (1944) reported a possible instance of homozygosity, 2 affected parents had a stillborn offspring who had extensive angiomatous malformation of the viscera.
In a large Arab family in the Sahara, Muller et al. (1978) found 87 cases in 6 generations. Because of the extensive consanguinity in the kindred, a person considered to be homozygous was identified. In the case of 4 couples indicated in the pedigree, both partners were affected. The son of one such couple had a total of 13 children by 4 different wives. All the wives were unaffected; all the children were affected. According to the Bayes theory, the probability of homozygosity was estimated to be 0.99975. The father, who was the presumed homozygote and also the proband, had severe but no exceptionally unusual manifestations of the disease.
MappingUsing microsatellite markers in a study of 2 extensively affected families, McDonald et al. (1993) showed that the HHT gene maps to 9q. D9S164 showed a combined maximum lod score of 4.39 at a recombination fraction of 0.14, and D9S103 showed a combined maximum lod score of 3.53 at a recombination fraction of 0.11. The probable location of the HHT gene, otherwise symbolized ORW, is 9q33-q34.1. McDonald et al. (1994) estimated that the closest marker, D9S65, is within 1 cM of the gene; it showed a combined lod score of 11.41 with HHT. Shovlin et al. (1994) independently assigned HHT to 9q.
Molecular GeneticsMcAllister et al. (1994) examined endoglin (ENG; 131195), a transforming growth factor-beta (TGF-beta) binding protein, as a candidate gene for HHT because of its chromosomal location, expression pattern, and function. They identified heterozygous mutations in the ENG gene (131195.0001-131195.0003) in 3 affected individuals from different families. This was the first human disease defined as due to a mutation in a member of the TGF-beta receptor complex. Primary pulmonary hypertension (PPH1; 178600) is another autosomal dominant inherited vascular disorder that is caused by a defect in BMPR2 (600799), which is a member of the TGF-beta signaling pathway.
In 160 unrelated cases of HHT, Lesca et al. (2004) screened the coding sequences of the ENG and ALK1 genes. Germline mutations were identified in 100 patients (62.5%); 36 of the mutations were in ENG and 64 were in ALK1.
Wehner et al. (2006) identified mutations in 32 (62.7%) of 51 unrelated German patients with HHT. Thirteen mutations were in the ENG gene, consistent with HHT1, and 17 mutations were in the ACVRL1 gene, consistent with HHT2. Analysis of genotype/phenotype correlations was consistent with a more common frequency of PAVMs in patients with HHT1.
Bossler et al. (2006) described the results of mutation analysis on a consecutive series of 200 individuals undergoing clinical genetic testing for HHT. A total of 127 probands were found, with sequence changes consisting of 103 unique alterations, 68 of which were novel. In addition 8 intragenic rearrangements in the ENG gene (131195), and 2 in ACVRL1 gene (601284) were identified. Surprisingly, almost 50% of the individuals with a single symptom were found to have a significant sequence alteration; 3 of these reported only nosebleeds.
In a German woman with clinical features of HHT and negative direct sequencing results, Shoukier et al. (2008) identified a deletion of exon 4 of the ENG gene using quantitative real-time polymerase chain reaction (qRT-PCR) and confirmed by multiplex ligation-dependent probe amplification (MLPA).
Exclusion Studies
Greenspan et al. (1995) excluded the COL5A1 gene as a candidate for HHT mapping to chromosome 9q.
Genotype/Phenotype CorrelationsBerg et al. (2003) performed a questionnaire-based study to delineate phenotypic differences between HHT1 and HHT2, which are caused by mutation in the ENG gene and ALK1 (601284) gene, respectively. The questionnaires were completed by 83 patients with known mutations (49 had HHT1 and 34 had HHT2). Patients with HHT1 reported an earlier onset of epistaxis and telangiectasis than those with HHT2. Pulmonary arteriovenous malformations were reported only in the group of HHT1 patients.
Among 14 kindreds with HHT1 and 12 with HHT2 confirmed by genetic analysis, Bayrak-Toydemir et al. (2006) found that HHT2 was associated with later onset and more hepatic involvement than HHT1.
Letteboer et al. (2006) analyzed phenotype in relation to sex in 584 Dutch probands and affected family members with HHT1 and HHT2 confirmed by genetic analysis. For the HHT1 group, they found a significantly higher prevalence of PAVM and hepatic AVM in women than in men.
In a study of 268 Dutch patients with HHT1 and 130 Dutch patients with HHT2, Letteboer et al. (2008) found that oral and nasal mucosal telangiectases were present earlier in life in patients with HHT1 compared to patients with HHT2, whereas dermal lesions were more frequent and appeared earlier in life in patients with HHT2. In both groups, telangiectases of the nasal mucosa were present at a higher prevalence and started to appear earlier in life than those of the oral mucosa or dermal sites. The number of sites affected increased with age in both groups. In patients with HHT1, more women than men had skin telangiectases, particularly on the face. These results confirmed that the frequency of AVMs differ between patients with HHT1 and HHT2, and that these differences can be detected on physical examination.
HeterogeneityGenetic heterogeneity was indicated by the results of linkage studies by several groups. Shovlin et al. (1994) found one family that did not map to 9q3. Porteous et al. (1994) pointed out that all of the previously reported 9q34-linked families contained at least 1 affected member with a symptomatic PAVM. Porteous et al. (1994) reported 4 families apparently unlinked to 9q34 and with no evidence of PAVMs. In a study by McAllister et al. (1994), 4 of 7 families gave a posterior probability of more than 99% being of the linked type and 3 families appeared unlinked to 9q34. They were impressed also by the absence of PAVMs in the 3 9q3-unlinked families. In 3 unrelated families of Dutch origin, Heutink et al. (1994) confirmed the linkage to 9q, and in a fourth unrelated family in which 'considerably fewer pulmonary arteriovenous malformations' were present, there was evidence for nonlinkage to this region.
Cronstedt et al. (1982) observed the coexistence of HHT disease and primary thrombocythemia (187950) in 2 patients, both men in their 70s.
The observation of a family with both type IIA von Willebrand disease (VWF2A; see 613554) and HHT prompted Iannuzzi et al. (1991) to study genetic linkage of the 2 conditions. No linkage was detected and the VWF gene (613160) was ruled out as a candidate gene for HHT because of the finding of segregation in linkage studies.
PathogenesisBraverman et al. (1990) reconstructed representative cutaneous telangiectases by computer from serial 1- or 2-mm plastic embedded sections. The earliest clinically detectable lesion was a focal dilatation of postcapillary venules, which continued to enlarge and eventually connect with dilated arterioles through capillaries. As the vascular lesion increased in size, the capillary segments disappeared and a direct arteriovenous communication was formed. This sequence of events was associated with a perivascular mononuclear cell infiltrate in which most of the cells were lymphocytes and a minority are monocytes/macrophages by ultrastructural characteristics. The telangiectatic lesions of scleroderma are also composed of dilated postcapillary venules and also associated with perivascular infiltrates. Cherry angiomas, however, which are produced by capillary loop aneurysms, are not associated with infiltrates.
DiagnosisAn important phenocopy is the CRST syndrome (calcinosis, Raynaud syndrome, sclerodactyly, telangiectasia; 181750), a probable 'collagen vascular disease.' The mucosal and cutaneous telangiectases are indistinguishable from those of the hereditary disorder (Winterbauer, 1964). Conlon et al. (1978) described 2 families in which telangiectasia like that of HHT disease occurred with von Willebrand disease. Other families with combined von Willebrand disease and HHT disease were described by Ramsay et al. (1976), Ahr et al. (1977), Hanna et al. (1984), and Iannuzzi et al. (1991). Since the CRST syndrome is occasionally familial (Frayha et al., 1977), a positive family history is not a conclusive differentiating feature of HHT disease.
On behalf of the Scientific Advisory Board of the HHT Foundation International, Inc., Shovlin et al. (2000) presented consensus clinical diagnostic criteria. The 4 criteria (epistaxes, telangiectasia, visceral lesions, and an appropriate family history) were carefully delineated. They considered the HHT diagnosis to be definite if 3 criteria were present. They suggested that a diagnosis of HHT cannot be established in patients with only 2 criteria, but should be recorded as possible or suspected in order to maintain a high index of clinical suspicion. If fewer than 2 criteria are present, HHT is unlikely, although children of affected individuals should be considered at risk in view of age-related penetrance in this disorder. They pointed out that these criteria may be refined as molecular diagnostic tests become available in the future.
Mager and Westermann (2000) used capillary microscopy to compare the capillary pattern of the fingernail folds in 54 patients with confirmed diagnoses of HHT and 40 healthy controls. Forty-five (83%) of the 54 patients with HHT had giant loops between the normal capillaries in the nail fold and 2 patients had enlargement of the draining limb of the capillary only. Seven patients (13%) had no vascular abnormalities in the nail fold. Seven of 9 patients with HHT but without cutaneous telangiectases had microvascular abnormalities. None of the volunteers had vascular abnormalities. The difference between both groups was significant (chi square, P less than 0.001). Mager and Westermann (2000) concluded that capillary microscopy can be useful in diagnosing HHT, especially in children with an affected parent and cases where there are few or atypical telangiectases present.
Clinical ManagementFlessa and Glueck (1977) recommended Enovid (a combination of a progestogen and an estrogen) for control of severe nosebleeds. They described experience with 9 patients of whom 1 was male. Vase (1981) could demonstrate no benefit of estrogen therapy. Oral estrogen has been found useful in controlling the frequency and severity of epistaxis (Harrison, 1982). It improves the continuity of telangiectatic endothelium and induces metaplasia of overlying epithelium (Menefee et al., 1975). Haq et al. (1988) used danazol, a synthetic weak androgen, with highly satisfactory results in a single patient, a 41-year-old man.
Aminocoporic acid, an antifibrinolytic drug, can reduce epistaxis in HHT (Saba et al., 1994), but its effect is inconsistent (Korzenik et al., 1994). Sabba et al. (2001) successfully treated 3 HHT patients with tranexamic acid, another antifibrinolytic drug which is 10 times as potent as aminocoporic acid and has a longer half-life. Klepfish et al. (2001) reported successful use of topical tranexamic acid for severe epistaxis in HHT.
White et al. (1988) reported embolotherapy of pulmonary arteriovenous malformations in 67 patients with HHT. Eleven of the patients had been discovered by means of family screening with measurements of arterial blood gases and chest radiography. Hypoxemia in the upright position is a clue to the presence of PAVMs. The AV fistulae are most often found in the lower lobes.
Lee et al. (1997) reported the long-term results of transcatheter embolotherapy of large pulmonary arteriovenous malformations in 221 consecutive patients, many of them with HHT, treated over a period of 18 years by a single physician, Robert I. White, Jr. The follow-up focused particularly on 45 patients with 52 PAVMs supplied by feeding arteries 8 mm in diameter or larger. Of these 45 patients, 38 (84%) with 44 PAVMs (85%) were cured by the first embolotherapy (mean follow-up, 4.7 years). Acute periprocedural complications included self-limited pleurisy (31%), angina secondary to air embolus (2%), and paradoxical embolization of a device during deployment (4%). None of these events led to short- or long-term sequelae. Seven patients (16%) had persistence of the PAVM, attributable to recanalization in 4 patients and to interim accessory artery growth in 3. Two of these patients presented with ischemic stroke several years after the initial treatment. Eight persistent PAVMs were re-treated successfully, 7 by a second procedure and 1 with a third procedure (mean follow-up, 5.9 and 5.3 years, respectively). Thus, embolotherapy was successful in a great majority of cases. Continued patency due to recanalization or accessory artery growth was easily detected and treated.
Bose et al. (2009) reported a 42-year-old man with a 3-generation family history of HHT who presented with longstanding epistaxis, hemoptysis, and a hemoglobin level half that of normal. After unsuccessful treatment with oral and intravenous iron, he received 4 cycles over 8 weeks of an anti-VEGF (see 192240) antibody, bevacizumab. After treatment, the patient's episodes of epistaxis were fewer in number and of shorter duration, and his hemoglobin level remained stable without transfusion.
Oosting et al. (2009) reported treatment with bevacizumab in a 55-year-old man with HHT who had intractable pain and frequent episodes of pancreatitis related to pancreatic AVMs. The treatment immediately stopped the patient's epistaxis, skin vascular signs became less pronounced, and the frequency and severity of pancreatitis diminished to the point where morphine and tube feeding could be discontinued. No change in the volume of AVMs was observed on CT scan. Retornaz et al. (2009) administered bevacizumab to a 65-year-old woman with HHT and life-threatening, recurrent hemorrhage, for which she had received 27 blood transfusions over a 6-month period. After treatment, blood transfusions were not required for 2 months; subsequently, hemorrhage recurred but with a reduced need for blood transfusion. Bose et al. (2009) noted that these cases provided further evidence of the efficacy of bevacizumab in patients with HHT, with improvement in symptoms and transfusion requirements without appreciable change in AVMs; they stated that although there was no difference in the size of their patient's pulmonary AVMs on CT scan before and after bevacizumab, he continued to report symptomatic benefit more than a year after completing therapy.
Lebrin et al. (2010) found that treatment with thalidomide, which has antiangiogenic activity, reduced nosebleed frequency in 6 of 7 individuals with HHT, and reduced the duration of nosebleeds in 3 of 4 for whom data were available. There were some side effects, including constipation and drowsiness. In vitro studies of mouse tissue showed that thalidomide stimulated the recruitment of mural cells to the vessel branches, resulting in a stabilization of blood vessels. Studies in Eng +/- mice also showed that thalidomide normalized inappropriate vessel formation and promoted pericyte and mural cell activation and vessel maturation via increased expression of Pdgfb (190040).
Brinkerhoff et al. (2011) described the long-term outcome of a patient who received multiple repeat courses of intravenous bevacizumab, a potent VEGF antagonist, for treatment of severe HHT. The patient was a 62-year-old male with severe HHT-related epistaxis who required blood transfusions and intravenous iron therapy to maintain a baseline hemoglobin level ranging from 5 to 7 grams per deciliter. Treatment with 4 intravenous infusions every 2 weeks resolved the epistaxis and improved his hemoglobin level to 13 grams per deciliter. After 1 year without treatment, he had a progressive relapse. Retreatment again resulted in cessation of epistaxis and a concomitant rise in hemoglobin. Subsequently a third course was required. In each case, there was a favorable response and no adverse events.
Population GeneticsIn a study of 18 families, Tuente (1964) estimated the frequency of the condition to be 1 or 2 in 100,000. The mutation rate was estimated to be 2 x 10(-6) to 3 x 10(-6).
Porteous et al. (1992) asked all clinicians in the northern region of England for information regarding their patients with HHT; 79 patients were identified in a population of 3.1 million, giving a minimum point prevalence of 1 in 39,216. Given the variable expression, the true incidence is likely to be much higher than this figure.
Plauchu et al. (1980) found a concentration of HHT patients in Haut-Jura in eastern France; 120 affected individuals from 42 families lived in a 300-km square area.
Bideau et al. (1992) reported that only 17.8% of the genes of inhabitants of the Valserine valley of the French Jura could be traced to the 'original population,' although persons affected with HHT disease belonged to a subset of the population that had lived in the villages for more than 10 generations. All patients in 85 sibships were related. The smallest number of originator couples who lived at the beginning of the 18th century amounted to 16; the unique originator may, therefore, have lived approximately 4 generations earlier.
Guttmacher et al. (1994) suggested that the prevalence of HHT has been underestimated at the level of 1 in 50,000 to 100,000 and that the disorder has not received the attention it deserves from the medical genetics community. He urged clinical geneticists and genetic counselors to play an active role in making the diagnosis, coordinating care, and providing genetic counseling. They estimated the minimal prevalence rate of HHT in Vermont to be 1:16,500 and suggested that this frequency is not atypical of rates elsewhere.
Dakeishi et al. (2002) estimated the population prevalence of HHT in the Akita prefecture of northern Japan to be 1:5,000 to 1:8,000, roughly comparable with those reported in European and U.S. populations, which is contradictory to the traditional view that HHT is rare among Asians.
Westermann et al. (2003) studied HHT in the Afro-Caribbean population of the Netherlands Antilles and found a point prevalence of 1 in 1,331 inhabitants older than 12 years, the highest known in the world.
HistoryOsler (1849-1919) described this disorder as a 'family form of recurring epistaxis, associated with multiple telangiectases of the skin and mucous membranes' (Osler, 1901). The only previous report he could find was that of Rendu dated 1896. Because of his prominence as a physician and author of a textbook, Osler 'put the disorder on the map.' F. Parkes Weber (1863-1962), who pronounced his name in the Germanic manner even though he was born in England and always lived there, described cases later as part of a lifelong interest in angiomas and other vascular lesions (McKusick, 1963). The frequent eponymic sequence, although not chronologically accurate, is perhaps justified by the contribution to the nosology of the entity: Osler-Rendu-Weber (pronounced OHz-ler, ren-DYU, and VAY-ber). Hanes (1909), then a medical resident at the Johns Hopkins Hospital, wrote a rather comprehensive discussion of this disorder, together with color illustrations of the lesions of the lips, tongue, and face, and named the disorder 'hereditary hemorrhagic telangiectasia.'
Christian (1949), who graduated from Johns Hopkins in 1900 during Osler's time there, wrote as follows: 'At another of the dispensary clinics it fell to my lot to demonstrate the case of a young man who frequently had come to the dispensary, as well as been a patient several times in the hospital wards. He was deeply jaundiced and had a large liver and many angiectases in his nose, which bled frequently and profusely. His condition had been diagnosed as Hanot's cirrhosis. His brother, a little older, had the same disease. The patient had devised a very simple way to control his nose bleeds: He took a thin rubber finger cot, put into its end a small cork, through which passed a small glass tube, and to the glass tube he had attached a bit of thin-walled rubber tubing. He would insert the finger cot well into his bleeding nostril, expand it by blowing through the rubber tubing and clamp off the tubing between his teeth to keep the cot distended until its pressure stopped the nosebleed. I had him demonstrate this to the section, while Dr. Osler commented on how simple but ingenious methods might be useful to the physician and patient....Dr. Osler had asked me to keep track of the patient, to report on his visits to the dispensary and to make follow-up visits at his home. At a later clinic Dr. Osler asked me how the patient was, and I replied, 'I think he is about as usual. I visited him about two weeks ago.' With this, Dr. Osler, to my embarrassment, dramatically brought forth a tray containing a large liver and other organs, saying, 'Christian, he did not continue to do so well. Dr. MacCallum autopsied him this morning.' That was the only liver showing Hanot's cirrhosis that I ever saw. Obviously, it made a great impression on me, and for the subsequent fifty years I have diligently sought for another patient with similar cirrhosis of the liver, so far with no success.' The description by Christian (1949) sounds much like that given by Osler (1901) in his classic paper but the latter concerned a man from Kentucky whom he first saw in 1896, who had no affected relatives and no sign of liver disease, and who was still alive at the time of Osler's report. Osler (1901) wrote: 'He sent a diagram of an ingenious arrangement. He took a rubber finger-stall about three inches long, into which was tied a small bit of rubber tubing, with a stop-cock at one end. He inserted the finger-stall, relaxed, then put the tubing in his mouth, inflated it, and turned the stop-cock.' The diagram was included in a letter dated Dec. 16, 1898. In the fifth edition of his Principles and Practice of Medicine (1904; p. 574), Osler wrote concerning Hanot hypertrophic cirrhosis: 'Of four recent cases under my care, the ages were from twenty to thirty-five. Two were brothers.' Hanot cirrhosis is a vague entity at best. Did the 2 brothers in fact suffer from Osler's disease, hereditary hemorrhagic telangiectasia (as it was designated by Hanes, 1909), which is known to be accompanied by cirrhosis?
Reported instances of familial epistaxis (e.g., Lane, 1916) probably represented this disorder. Indeed, Osler (1901) entitled his original report, 'A family form of recurring epistaxis.'
Fuchizaki et al. (2003) provided biographical information on the individuals whose names are included in triple eponym Rendu-Osler-Weber.
A comment on semantics: The individual lesion in HHT is a telangiectasis (pl., telangiectases); the process is telangiectasia. Multiple lesions should not be referred to as 'telangiectasias.' One would use the latter term only in a statement such as, 'Dr. William Bennett Bean was a student of the telangiectasias.'
Animal ModelLi et al. (1999) generated mice deficient for endoglin (131195) using homologous recombination. Eng +/- mice had normal life expectancy, fertility, and gross appearance. Eng -/- mice died by embryonic day 11.5. At embryonic day 10.5, Eng -/- mice were 3 times smaller than Eng +/+ mice and had fewer somites. The Eng -/- embryos exhibited an absence of vascular organization and the presence of multiple pockets of red blood cells on the surface of the yolk sac. Epithelial marker expression was not disrupted in Eng -/- mice. There was persistence of an immature perineural vascular plexus, indicating a failure of endothelial remodeling in Eng -/- embryos. At embryonic day 10.5, the cardiac tube did not complete rotation and was associated with a serosanguinous pericardial effusion. By embryonic day 10.5, the major vessels including the dorsal aortae, intersomitic vessels, branchial arches, and carotid arteries were atretic and disorganized in Eng -/- embryos. There was also poor vascular smooth muscle cell formation at both embryonic days 9.5 and 10.5. These vascular smooth muscle cell abnormalities preceded the differences in endothelial organization. In contrast to mice lacking TGF-beta, vasculogenesis was unaffected. Li et al. (1999) concluded that their results demonstrated that endoglin is essential for angiogenesis and suggest a pathogenic mechanism for HHT1.