Exostoses, Multiple, Type Ii
A number sign (#) is used with this entry because multiple exostoses type II (EXT2) is caused by heterozygous mutation in the gene encoding exostosin-2 (EXT2; 608210) on chromosome 11p11.
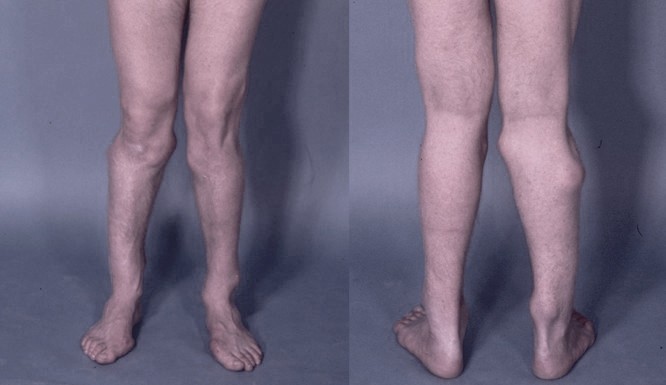
DescriptionHereditary multiple exostoses is an autosomal dominant disorder characterized by multiple exostoses most commonly arising from the juxtaepiphyseal region of the long bones.
For a general phenotypic description and a discussion of genetic heterogeneity of multiple exostoses, see EXT1 (133700).
MappingCook et al. (1993) concluded that about 70% of multiple exostoses families show linkage to markers in the 8q24.11-q24.13 region (EXT1). Multiple exostoses in the other families appears to be caused by a mutation at another locus, unlinked to markers in that region. Investigating 2 large exostoses pedigrees in which linkage to markers from 8q24 was excluded, Wu et al. (1994) found evidence of linkage to microsatellite markers from the proximal short and long arms of chromosome 11. The highest lod score by 2-point analysis was found with D11S554; maximum lod = 7.148 at theta = 0.03.
Hecht et al. (1995) reported a large multigenerational family with multiple exostosis who demonstrated linkage of the disease to chromosome 11 markers. One family member had a chondrosarcoma. Constitutional and tumor DNAs from the family member with chondrosarcoma were compared using short tandem repeat (STR) markers from chromosomes 8, 11, and 19. Loss of heterozygosity (LOH) in the tumor was observed for chromosome 8 and 11 markers, but chromosome 19 markers were intact. Hecht et al. (1995) observed an apparent deletion of D11S903 in constitutional DNA from all affected individuals and in the tumor sample. These results indicated that the EXT2 gene maps to the region containing D11S903, which is flanked by D11S1355 and D11S1361.
Studying 7 extended multiple exostoses families, all linked to the EXT2 locus, Wuyts et al. (1995) refined the localization of the EXT2 gene to a 3-cM region flanked by D11S1355 and D11S1361/D11S554. The findings indicated that the EXT2 gene is located on 11p12-p11. The refined localization excluded a number of putative candidate genes located in the pericentromeric region of chromosome 11. Blanton et al. (1996) studied 12 large multigenerational EXT families and found that the disorder mapped to 8q24 in 6 and to 11p in 6. The authors noted that the 2 sets of families were clinically indistinguishable. None of the families mapped to the chromosome 19 locus.
McGaughran et al. (1995) described a patient with the combination of multiple exostoses and the WAGR syndrome (Wilms tumor, aniridia, genital anomalies, and mental retardation; 194070), a well-documented contiguous gene syndrome resulting from deletion of 11p13. Their patient showed a del(11)(p14.2p11.2). As pointed out by Potocki et al. (1995), the description of the contiguous gene syndrome resulting from interstitial deletion of 11p, del(11)(p12p11.2), including multiple exostoses as a feature, provided confirmation of the mapping of EXT2. Other features of this contiguous gene syndrome are mental retardation and parietal foramina, known as Catlin marks (168500). Potocki and Shaffer (1996) reported the clinical and molecular findings in another patient with an 11(p12p11.2) deletion. Cytogenetic and molecular analysis demonstrated a de novo, paternally-derived deletion for markers known to be tightly linked to EXT2. The patient had an unusual facies (bilateral epicanthal folds, ptosis, short philtrum, and downturned upper lip), mental retardation, multiple exostoses, brachycephaly, and bilateral parietal foramina.
Molecular GeneticsIn a family with multiple exostoses, Stickens et al. (1996) identified a 4-bp deletion in the EXT2 gene (608210.0001), resulting in a premature stop codon and truncated gene product. Stickens et al. (1996) speculated that a second mutation event was necessary for the development of exostoses, thus accounting for the asymmetry of exostoses observed in the long bones.
In 2 families with multiple exostoses, Wuyts et al. (1996) identified 2 different mutations in the EXT2 gene: a nonsense mutation (608210.0002) and a splice site mutation (608210.0003). In 5 of 17 (29%) families with hereditary multiple exostoses, Philippe et al. (1997) identified 4 mutations in the EXT2 gene, including a missense mutation (608210.0004) and 3 alterations that resulted in premature stop codons. Seven (41%) of the families had mutations in the EXT1 gene.
Of 26 EXT families originating from 9 countries, Wuyts et al. (1998) found that 10 families had an EXT1 mutation and 10 had an EXT2 mutation. Twelve of these mutations had not previously been described. From a review of these and previously reported mutations, Wuyts et al. (1998) concluded that mutations in either the EXT1 or the EXT2 gene are responsible for most cases of multiple exostoses. Most of the mutations in these 2 genes cause premature termination of the EXT proteins, whereas missense mutations are rare. The authors concluded that the development of exostoses is mainly due to loss of function of EXT genes, consistent with the hypothesis that the EXT genes have a tumor suppressor function.
Wuyts and Van Hul (2000) stated that 49 different EXT1 and 25 different EXT2 mutations had been identified in patients with multiple exostoses, and that mutations in these 2 genes were responsible for over 70% of the EXT cases.
Genotype/Phenotype CorrelationsFrancannet et al. (2001) identified mutations in 36 of the 38 families linked to EXT1 or EXT2. No mutations were found in 2 EXT1-linked families. Nine of the mutations occurred in the EXT2 gene. A severe phenotype ('S') was shown to be significantly associated with EXT1 mutations, whereas a moderate phenotype ('M') was associated with EXT2 mutations. One subgroup of the S phenotype, IS (10 to 25 exostoses, no vertebral exostoses, height below the 10th centile), was associated with mutations in EXT1 or EXT2. Mutations associated with another S subgroup, IVS (very short stature), were located in exon 1 of EXT1. Chondrosarcomas were found only in patients with EXT1 mutations.
In 7 patients with EXT1 mutations and 16 patients with EXT2 mutations, Alvarez et al. (2006) analyzed the anatomic burden of disease by clinical and radiographic examination and evaluation of 76 phenotypic parameters. Patients with EXT1 mutation were found to have more exostoses, more limb malalignment with shorter limb segments and height, and more pelvic and flatbone involvement.
Heinritz et al. (2009) identified 9 different mutations in the EXT2 gene in 11 of 23 German patients with multiple exostoses. Eleven other patients had mutations in the EXT1 gene; 1 patient had no detectable mutations. Among the EXT2 mutations, there were 3 recurrent mutations, Q172X (608210.0002), D227N (608210.0004), and Q258X (608210.0006), and 6 novel mutations (see, e.g., 608210.0007). Multiple splice site defects were identified. Although clinical details were limited, those with EXT1 mutations tended to have a more severe phenotype.