Grn Frontotemporal Dementia
Summary
Clinical characteristics.
The spectrum of GRN frontotemporal dementia (GRN-FTD) includes the behavioral variant (bvFTD), primary progressive aphasia (PPA; further subcategorized as progressive non-fluent aphasia [PNFA] and semantic dementia [SD]), and movement disorders with extrapyramidal features such as parkinsonism and corticobasal syndrome (CBS). A broad range of clinical features both within and between families is observed. The age of onset ranges from 35 to 87 years. Behavioral disturbances are the most common early feature, followed by progressive aphasia. Impairment in executive function manifests as loss of judgment and insight. In early stages, PPA often manifests as deficits in naming, word finding, or word comprehension. In late stages, affected individuals often become mute and lose their ability to communicate. Early findings of parkinsonism include rigidity, bradykinesia or akinesia (slowing or absence of movements), limb dystonia, apraxia (loss of ability to carry out learned purposeful movements), and disequilibrium. Late motor findings may include myoclonus, dysarthria, and dysphagia. Most affected individuals eventually lose the ability to walk. Disease duration is three to 12 years.
Diagnosis/testing.
The diagnosis of GRN-FTD is established in a proband with suggestive findings and a heterozygous pathogenic variant in GRN identified by molecular genetic testing.
Management.
Treatment of manifestations: Behavioral manifestations such as apathy, impulsivity, and compulsiveness may respond to selective serotonin reuptake inhibitors. Roaming, delusions, and hallucinations may respond to antipsychotic medications. Reports have suggested potential benefits with certain pharmacotherapy on management of FTD; however, evidence from randomized controlled trials is limited. Small-scale studies have suggested that trazodone may be helpful for treating irritability, agitation, depression, and eating disorders; methylphenidate and dextro-amphetamine may help minimize risk-taking behavior. Cholinesterase inhibitors examined in clinical trials were generally well tolerated: galantamine was used to treat PPA with stabilization of symptoms; rivastigmine was used to treat behavioral manifestations and appeared to decrease caregiver burden. Two open-label studies of memantine, an NMDA partial agonist-antagonist, demonstrated some efficacy on frontal behavior in those with bvFTD and improvement in cognitive performance in those with PPA-PNFA.
Genetic counseling.
GRN-FTD is inherited in an autosomal dominant manner. About 95% of individuals diagnosed with GRN-FTD have an affected parent. The proportion of affected individuals with a de novo GRN pathogenic variant is unknown but is estimated to be 5% or fewer. Each child of an individual with GRN-FTD has a 50% chance of inheriting the pathogenic variant. Once a GRN pathogenic variant has been identified in an affected family member, prenatal testing for a pregnancy at increased risk and preimplantation genetic testing are possible.
Diagnosis
Suggestive Findings
GRN frontotemporal dementia (GRN-FTD) should be suspected in individuals with the following clinical presentations and neuroimaging findings.
Clinical Presentations
Clinical presentations of GRN-FTD vary widely both among and within families and may resemble behavioral variant FTD (bvFTD), primary progressive aphasia (PPA), atypical parkinsonism, or corticobasal syndrome.
Behavioral variant FTD [Rascovsky et al 2011]
- Early behavioral disinhibition (including one of the following):
- Socially inappropriate behavior
- Loss of manners or decorum
- Impulsive, rash, or careless actions
- Early apathy or inertia (one of the following):
- Apathy
- Inertia
- Early loss of sympathy or empathy (one of the following):
- Diminished response to other people's needs and feelings
- Diminished social interest, interrelatedness, or personal warmth
- Early perseverative, stereotyped, or compulsive/ritualistic behavior (one of the following):
- Simple repetitive movements
- Complex, compulsive, or ritualistic behaviors
- Stereotypy of speech
- Hyperorality and dietary changes (one of the following):
- Altered food preferences
- Binge eating, increased consumption of alcohol or cigarettes
- Oral exploration or consumption of inedible objects
- Neuropsychological profile: executive/generation deficits with relative sparing of memory and visuospatial functions (all of the following):
- Deficits in executive tasks
- Relative sparing of episodic memory
- Relative sparing of visuospatial skills
Primary progressive aphasia (PPA). PPA has been further classified into three subtypes [Gorno-Tempini et al 2011]:
- Progressive nonfluent aphasia (PNFA, also known as nonfluent or agrammatic subtype of PPA)
- Semantic dementia (SD)
- Logopenic variant (logopenic PPA)Note: To date, the logopenic variant has not been associated with GRN-FTD.
The majority of the literature describes PNFA to be the predominant form of PPA in GRN-FTD, although there are a few reports of the SD phenotype as well.
The currently proposed diagnostic algorithm for PNFA requires a two-step process. First, individuals must meet the criteria for PPA, and after the diagnosis of PPA is established, the main features of the speech and language abnormalities may be considered to subcategorize into each of the PPA variants.
The diagnostic criteria of PPA [Mesulam 2001]:
- The most prominent clinical feature is difficulty with language.
- Language deficits are the principal cause of impaired daily living activities.
- Aphasia is the most prominent deficit at symptom onset and for the initial phases of the disease.
Note: The pattern of deficits cannot be accounted for by other nondegenerative diseases of the nervous system, medical disorders, or psychiatric diagnoses.
PPA subtypes
- Nonfluent variant of PPA (PPA-PNFA). The diagnostic criteria of PPA-PNFA include clinical presentation of aphasia with [Gorno-Tempini et al 2011]:
- At least one of the following core features:
- Agrammatism in language production
- Effortful, halting speech with inconsistent speech sound errors and distortions (apraxia of speech)
- At least two of the three following supportive features:
- Impaired comprehension of syntactically complex sentences
- Spared single-word comprehension
- Spared object knowledge
- Semantic variant of PPA (PPA-SD). The diagnostic criteria of PPA-SD require the presence of both of the following core features:
- Impaired confrontation naming
- Impaired single-word comprehension
AND at least three of the following four additional diagnostic features:- Impaired object knowledge, particularly for low frequency or low-familiarity items
- Surface dyslexia or dysgraphia
- Spared repetition
- Spared speech production (grammar and motor speech)
Atypical parkinsonism. Clinical features include the following:
- Bradykinesia
- Rigidity
- Gait instability
- Resting tremor
Corticobasal syndrome. Clinical features include the following [Armstrong et al 2013]:
- Progressive asymmetric rigidity
- Apraxia
- Alien-limb phenomenon
- Cortical sensory loss
- Focal dystonia
- Myoclonus
- Dementia
Neuroimaging
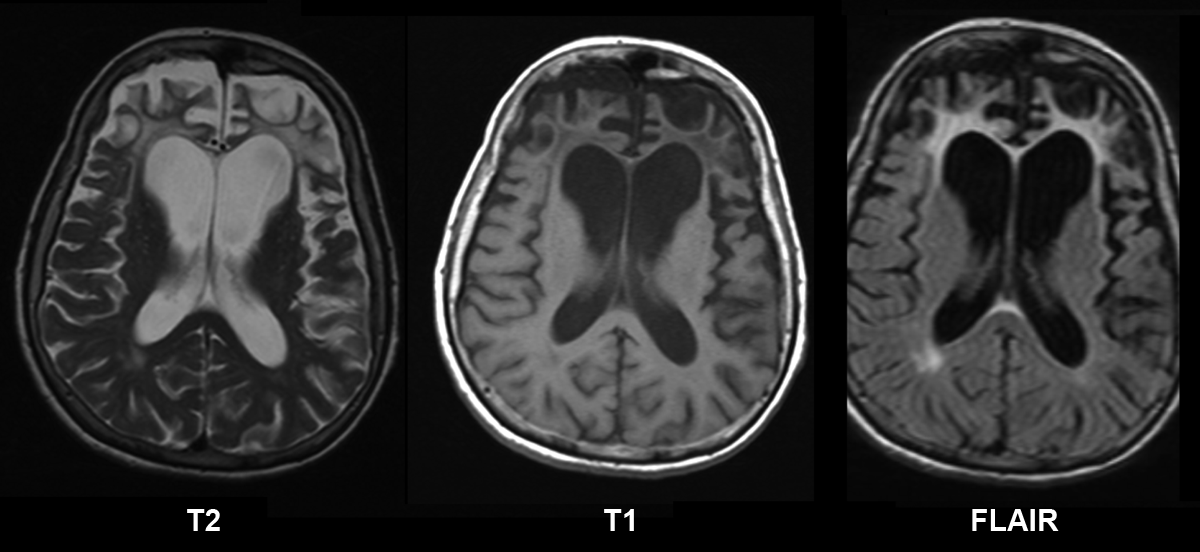
Computed tomography (CT) or magnetic resonance imaging (MRI) may show focal, often asymmetric atrophy in the frontal, temporal, and/or parietal lobes [Rohrer & Warren 2011]. Volumetric studies comparing the rate of brain atrophy between GRN-FTD and MAPT-FTD showed that individuals with GRN-FTD have a higher rate of whole-brain atrophy (3.5% per year) than those with MAPT-related FTD [Whitwell et al 2011].
Single photon emission computed tomography (SPECT) may reveal decreased perfusion in the frontal and temporal lobes [Pasquier et al 2003]. There is also evidence of poor cerebral perfusion in both anterior parietal lobes, predominantly on the left hemisphere and on the right inferior parietal cortex [Le Ber et al 2008].
Positron emission tomography (PET) may demonstrate decreased glucose metabolism in the frontal and temporal regions in the presymptomatic stage prior to structural changes [Jacova et al 2013, Caroppo et al 2015].
Establishing the Diagnosis
The diagnosis of GRN frontotemporal dementia (GRN-FTD) is established in a proband with a heterozygous pathogenic variant in GRN by molecular genetic testing (see Table 1).
Gene-targeted testing (multigene panel) requires that the clinician determine which gene(s) are likely involved, whereas comprehensive genomic testing does not. Because the phenotype of GRN-FTD is broad, individuals with the distinctive findings described in Suggestive Findings are likely to be diagnosed using gene-targeted testing (see Option 1), whereas those in whom the diagnosis of GRN-FTD has not been considered are more likely to be diagnosed using genomic testing (see Option 2).
Option 1
A frontotemporal dementia multigene panel that includes GRN and other genes of interest (see Differential Diagnosis) is most likely to identify the genetic cause of the condition at the most reasonable cost while limiting identification of variants of uncertain significance and pathogenic variants in genes that do not explain the underlying phenotype. Note: (1) The genes included in the panel and the diagnostic sensitivity of the testing used for each gene vary by laboratory and are likely to change over time. (2) Some multigene panels may include genes not associated with the condition discussed in this GeneReview. (3) In some laboratories, panel options may include a custom laboratory-designed panel and/or custom phenotype-focused exome analysis that includes genes specified by the clinician. (4) Methods used in a panel may include sequence analysis, deletion/duplication analysis, and/or other non-sequencing-based tests. For GRN-FTD a multigene panel that also includes deletion/duplication analysis is recommended (see Table 1).
For an introduction to multigene panels click here. More detailed information for clinicians ordering genetic tests can be found here.
Option 2
Comprehensive genomic testing. Exome sequencing is most commonly used; genome sequencing is also possible. If exome sequencing is not diagnostic – and particularly when evidence supports autosomal dominant inheritance – exome array (when clinically available) may be considered to detect (multi)exon deletions or duplications that cannot be detected by sequence analysis.
For an introduction to comprehensive genomic testing click here. More detailed information for clinicians ordering genomic testing can be found here.
Table 1.
Molecular Genetic Testing Used in GRN Frontotemporal Dementia
| Gene 1 | Method | Proportion of Probands with a Pathogenic Variant 2 Detectable by Method |
|---|---|---|
| GRN | Sequence analysis 3 | ~98.5% 4 |
| Gene-targeted deletion/duplication analysis 5 | ~1.5% 4, 6 |
- 1.
See Table A. Genes and Databases for chromosome locus and protein.
- 2.
See Molecular Genetics for information on allelic variants detected in this gene.
- 3.
Sequence analysis detects variants that are benign, likely benign, of uncertain significance, likely pathogenic, or pathogenic. Pathogenic variants may include small intragenic deletions/insertions and missense, nonsense, and splice site variants; typically, exon or whole-gene deletions/duplications are not detected. For issues to consider in interpretation of sequence analysis results, click here.
- 4.
Cruts et al [2006], Chen-Plotkin et al [2011], Van Langenhove et al [2013], Pottier et al [2018]. Note: Pottier et al [2018] identified 449 affected individuals with GRN disease-associated variants detected by sequence and deletion/duplication analysis in the ascertainment step of a genome-wide association study (see Pottier et al [2018], Supplementary Table 2).
- 5.
Gene-targeted deletion/duplication analysis detects intragenic deletions or duplications. Methods used may include quantitative PCR, long-range PCR, multiplex ligation-dependent probe amplification (MLPA), and a gene-targeted microarray designed to detect single-exon deletions or duplications. Gene-targeted deletion/duplication testing will detect deletions ranging from a single exon to the whole gene; however, breakpoints of large deletions and/or deletion of adjacent genes (e.g., those described by Milan et al [2017]) may not be detected by these methods.
- 6.
Gijselinck et al [2008], Pickering-Brown et al [2008], Rovelet-Lecrux et al [2008], Finch et al [2009], Chen-Plotkin et al [2011], Rohrer et al [2013], Van Langenhove et al [2013], Clot et al [2014]
Clinical Characteristics
Clinical Description
GRN frontotemporal dementia (GRN-FTD) generally affects the frontal and temporal cortex leading to behavioral changes, executive dysfunction, and language disturbances. In GRN-FTD, the parietal cortex and basal ganglia may be affected as well, resulting in parkinsonism, cortical basal syndrome, and memory impairment [Baker et al 2006, Masellis et al 2006, Mukherjee et al 2006, Behrens et al 2007, Josephs et al 2007, Mesulam et al 2007, Spina et al 2007].
Age of onset. The age of onset of GRN-FTD ranges from 35 to 87 years with a mean of 64.9 ± 11.3 years [Bruni et al 2007, Le Ber et al 2007, Rademakers et al 2007, Chen-Plotkin et al 2011].
Comparison studies demonstrate that onset age in individuals with GRN-FTD does not differ significantly from that in individuals without an identified GRN pathogenic variant [Beck et al 2008, Pickering-Brown et al 2008], while some studies suggested a younger onset age in those with GRN-FTD [Huey et al 2006, Davion et al 2007].
Neurocognitive symptoms. Neuropsychological testing may demonstrate early impairment on frontal lobe tasks or specific language dysfunction prior to the onset of frank dementia.
Behavioral disturbances are the most common early feature, followed by progressive aphasia [Gass et al 2006, Josephs et al 2007]. This is usually an insidious but profound change in personality and conduct, characterized by distractibility, loss of initiative, apathy, and loss of interest in their environment, often accompanied by neglect in personal hygiene and social disinhibition. Some affected individuals demonstrate impulsiveness or compulsiveness and may alter their eating habits with food fads and food craving.
With impairment in executive function, there is loss of judgment and insight, which may manifest early in the disease course as, for example, making poor financial decisions, quitting jobs abruptly, or becoming unduly forward or rude to strangers. Alternatively, persons with predominant apathy may lose all interest and initiative with usual activities, appear socially withdrawn, give up all previous hobbies and interests, and be unable to complete tasks due to lack of persistence. Early in the course of the illness, affected individuals may be misdiagnosed as having psychiatric conditions such as depression, mania, or psychosis because of the unusual and bizarre nature of their behavior. Psychometric testing may demonstrate impairment on frontal executive tasks including the Trail-Making Test, proverb interpretation, descriptions of similarities, categorical naming, and abstract pattern recognition (e.g., Wisconsin Card Sort Test).
Language deficits. Primary progressive aphasia (PPA), particularly the progressive non-fluent aphasia (PNFA) variant, can be another presentation of GRN-FTD [Mesulam et al 2007]. In early stages, PPA-PNFA often manifests as deficits in naming, word finding, or word comprehension. Although behavioral manifestations tend to be more common than language deficits as the initial presentation of GRN-FTD, in one series 82% of affected individuals eventually developed language problems [Josephs et al 2007, Caso et al 2012].
In contrast with PPA-PNFA, semantic dementia is characterized by impaired naming and comprehension, semantic paraphasias, and impaired recognition of familiar faces or objects. Although rare in GRN-FTD, pure semantic dementia (PPA-SD) has been described in a few studies [Whitwell et al 2007, Beck et al 2008]. In late stages, individuals with PPA-SD may develop impaired face recognition and behavioral changes including disinhibition and compulsion [Seeley et al 2005].
A number of studies have reported individuals with GRN-FTD who have presented with amnestic mild cognitive impairment, which may be mistaken for Alzheimer disease [Carecchio et al 2009, Kelley et al 2010].
Movement disorders. In several families with GRN-FTD, parkinsonism is prominent, and in some the initial clinical diagnosis was corticobasal syndrome [Gass et al 2006, Masellis et al 2006, Benussi et al 2009, Moreno et al 2009]. Early findings include rigidity, bradykinesia or akinesia (slowing or absence of movements), limb dystonia, apraxia (loss of ability to carry out learned purposeful movements), and disequilibrium. Late motor findings may include myoclonus, dysarthria, and dysphagia. Most affected individuals eventually lose the ability to walk.
Motor neuron disease. Although the histopathologic findings of ubiquitin-positive inclusions were initially associated with motor neuron disease, it appears to occur only rarely (if at all) in GRN-FTD [Schymick et al 2007].
Disease course. The mean age at death is 65±8 years. Disease duration ranges from three to 12 years [Gass et al 2006].
Neuropathology. The neuropathology of GRN-FTD is characterized by the following [Mackenzie et al 2006, Mackenzie et al 2011]:
- Tau-negative alpha-synuclein-negative ubiquitin-positive "cat-eye" or lentiform-shaped neuronal intranuclear inclusions (NII), often found in the neocortex and striatum
- Superficial laminar spongiosis with ubiquitin-positive neurites and neuronal cytoplasmic inclusions (NCI) in the neocortex
- Granular appearance of the ubiquitin-immunoreactive (ub-ir) neurites in the striatum and the NCI in the hippocampus
- Phosphorylation of S409/410 of TDP-43 in pathologic inclusions [Neumann et al 2009]The major protein component of these ubiquitin inclusions is a TAR DNA-binding protein of 43 kd (TDP-43). TDP-43 is a nuclear factor involved in regulating transcription and alternative splicing [Arai et al 2006, Neumann et al 2006]. It is mostly a nuclear protein, although recent studies have shown that it shuttles between the nucleus and cytoplasm in normal conditions [Ayala et al 2008]. While its physiologic function remains unclear, it has been demonstrated to bind to a large number of RNA targets with a preference for UG-rich intronic regions and is important in many vital cellular processes [Sendtner 2011].
It is now recognized that pathologically, GRN-FTD is a major subtype of frontotemporal lobar degeneration (FTLD). The neuropathologic diagnostic criteria for FTLD have been updated based on current molecular understanding of the disease [Mackenzie et al 2011].
Genotype-Phenotype Correlations
No obvious correlations between age of onset, disease duration, or clinical phenotype and specific GRN pathogenic variants have been identified. Clinical variability is high among individuals with the same GRN pathogenic variant.
Penetrance
Penetrance of GRN-FTD is about 90% by age 75 years, but apparent reduced penetrance has also been observed on occasion [Cruts et al 2006, Gass et al 2006].
A study of the common p.Arg493Ter pathogenic variant showed that 60% of individuals with this variant were affected by age 60 years, and more than 95% were affected by age 70 years [Rademakers et al 2007]. Age at onset of frontotemporal lobar degeneration (FTLD) was younger in individuals with a GRN pathogenic variant vs those without one (median: 58.0 vs 61.0 years), as was age at death (median: 65.5 vs 69.0 years) [Chen-Plotkin et al 2011].
In a large series in France, 3.2% of simplex cases (i.e., only one affected individual in a family) with FTD were found to have a GRN pathogenic variant, suggesting possible de novo variant or incomplete penetrance [Le Ber et al 2007].
Nomenclature
The term frontotemporal dementia (FTD) is used in this GeneReview to designate the clinical presentation of the dementing illness, while the term frontotemporal lobar degeneration (FTLD) is used to denote the pathologic diagnosis of the disease.
Note that PGRN, the earlier designation for the gene GRN, may be used in the literature as well (e.g., PGRN-FTD).
Prior to the identification of GRN as the gene in which a pathogenic variant is responsible for this form of FTD, a number of terms were used to describe this disorder.
- FTDU-17. Analogous to FTDP-17, the term "FTDU-17" has been used because the pathologic characteristics of this condition are associated with ubiquitinated inclusions and the genetic locus was also located on chromosome 17.
- HDDD1 and HDDD2. Familial dementia in other kindreds with similar clinical presentations was descriptively named hereditary dysphasic disinhibition dementia (HDDD1 and HDDD2). It has now been shown that GRN pathogenic variants are also responsible for the phenotype in these families, and therefore these are now considered GRN-FTD [Mukherjee et al 2006, Behrens et al 2007].
Prevalence
Frontotemporal dementia (FTD) accounts for 5%-10% of all individuals with dementia and 10%-20% of individuals with dementia with onset before age 65 years [Bird et al 2003].
GRN-FTD represents about 5% of all FTD, and 20% of FTD in which the family history is positive.
Genetically Related (Allelic) Disorders
Individuals with biallelic GRN pathogenic variants and the phenotype of neuronal ceroid lipofuscinosis, a lysosomal storage disease that is strikingly different from FTD, have been reported [Smith et al 2012, Kamate et al 2019]. This finding further highlights the role of GRN in lysosomal function and regulation (see Molecular Genetics).
Differential Diagnosis
Neuroimaging can evaluate for other conditions that mimic frontotemporal dementia (FTD) (e.g., white matter diseases, frontotemporal focal lesions, frontal lobe tumors, and cerebrovascular disease).
The clinical manifestations of GRN-FTD significantly overlap with those of other conditions including FTD with or without parkinsonism associated with pathogenic variants in MAPT (OMIM 600274), Parkinson disease, Alzheimer disease, Pick disease (OMIM 172700), other inherited FTD disorders, corticobasal degeneration, progressive supranuclear palsy, and Creutzfeldt-Jacob disease (OMIM 123400). This clinical overlap makes it difficult to predict which family has a GRN pathogenic variant by clinical presentation alone.
Up to 50% of individuals with FTD have a positive family history of dementia, usually with autosomal dominant inheritance. Table 2 below lists the most common genes associated with familial FTD.
Table 2.
Genes in the Differential Diagnosis of GRN Frontotemporal Dementia
| Gene(s) | Differential Diagnosis Disorder | Clinical Features of Differential Diagnosis Disorder | |||
|---|---|---|---|---|---|
| Onset | Disease Duration | Pathology | Comment | ||
| Most commonly involved genes | |||||
| C9orf72 | ALS & FTD | Mean: 54.3 yrs; range: 34-74 yrs | Mean: 5.3 yrs; range: 1-16 yrs | TDP-43 pathology is found in a wide neuroanatomic distribution, w/particular involvement in extramotor neocortex & hippocampus & in lower motor neurons | May be misdiagnosed as bvFTD, PPA-PNFA, or ALS. 1 Heterogeneity in clinical presentation is common w/in families. Phenotypes tend to converge w/disease progression. |
| MAPT | FTDP-17 (OMIM 600274) | Usually age 40-60 yrs; may occur earlier or later | Usually 5-10 yrs; may be up to 20-30 yrs | At autopsy, all persons w/FTDP-17 show tau-positive inclusion pathology, whereas all persons w/GRN-FTD show ub-ir neuronal intranuclear inclusions. 2 | Presenile dementia affecting frontal & temporal cortex & some subcortical nuclei. Variable presentation; may present w/slowly progressive behavioral changes, language disturbances, &/or extrapyramidal signs; progresses over a few yrs to profound dementia w/mutism. 25%-40% of families w/AD FTD have mutation of MAPT. |
| Less commonly involved genes | |||||
| CHMP2B | FTD-3 | Typically in late 50s | Neuropathology assoc w/ubiquitin-positive but TDP-43- & FUS-negative inclusions | Usually presents w/a frontal lobe syndrome, parkinsonism, dystonia, pyramidal signs. Myoclonus may occur later in disease course. | |
| TARDBP | ALS or ALS w/FTD (see TARDBP-ALS) | 41-60 yrs | 2-4 yrs | TDP-43 inclusions in upper & lower motor neurons & cortex | Assoc w/~3% of familial ALS & occasionally FTD w/ALS |
| VCP | Inclusion body myopathy w/Paget disease of bone & FTD (IBMPFD) | Muscle disease & PDB: age 42 yrs; FTD: age 55 yrs | Numerous intranuclear & infrequent # of neuronal cytoplasmatic inclusions & dystrophic neuritis seen in neuropathology | Adult-onset proximal & distal muscle weakness (clinically LGMD 3), early-onset PDB 4, & FTD. Early-stage FTD: dysnomia, dyscalculia, comprehension deficits, paraphasic errors, & relative preservation of memory. Later stages: inability to speak, auditory comprehension deficits for even 1-step commands, alexia, & agraphia | |
AD= autosomal dominant; ALS = amyotrophic lateral sclerosis; FTD = frontotemporal dementia; bvFTD = behavioral variant FTD; FTDP = frontotemporal dementia with parkinsonism; FUS = fused in sarcoma; LGMD = limb-girdle muscular dystrophy; PNFA = progressive nonfluent aphasia; PDB = Paget disease of bone; PPA = primary progressive aphasia
- 1.
See Amyotrophic Lateral Sclerosis Overview.
- 2.
Ghetti et al [2003], Mackenzie [2007]
- 3.
Muscle weakness progresses to involve other limb & respiratory muscles; cardiac failure & cardiomyopathy have been observed in later stages of IBMPFD.
- 4.
Paget disease of bone (PDB) involves focal areas of increased bone turnover that typically lead to spine and/or hip pain and localized enlargement and deformity of the long bones.
Management
Evaluations Following Initial Diagnosis
To establish the extent of disease and needs in an individual diagnosed with GRN frontotemporal dementia (GRN-FTD), the following evaluations (if not performed as part of the evaluation that led to the diagnosis) are recommended:
- Detailed general, neurologic, and family history
- Physical examination
- Neurologic examination
- Cognitive examination. When clinical cognitive assessments are not informative enough, a neuropsychological assessment may be performed to provide a more comprehensive and objective view of a patient's cognitive function. Formal neuropsychological assessment requires comparison of the patient's raw score on a specific test to a large general population normative sample which is usually drawn from a population comparable to that of the person being examined. This allows for the patient's performance to be compared to a suitable control group, adjusted for age, gender, level of education, and/or ethnicity. While much more sensitive than bedside clinical cognitive examination, such assessment is resource intensive and time consuming.
- Discussion of capabilities for job and for driving
- Discussion of advanced care planning
- Consultation with a clinical geneticist and/or genetic counselor
Treatment of Manifestations
There is currently no known treatment for GRN-FTD or FTD in general. Psychosocial support is essential in the management of FTD and should include occupational therapy and environmental and physical interventions.
However, some behavioral manifestations such as apathy, impulsivity, and compulsiveness may respond to selective serotonin reuptake inhibitors. Behavioral changes and the loss of insight and judgment in individuals with GRN-FTD often present a considerable burden for caregivers. Information about the disease and psychological support for partners or other caregivers is essential. Caregiver support groups are valuable.
The behavioral and psychological manifestations should be treated as in other types of FTD. There is no consensus treatment guideline for GRN-FTD. In clinical practice those affected individuals who have very aggressive behavior have proven quite difficult to treat and have in some instances been treated with high doses of antipsychotics and/or antidepressants in order to relieve the physical aggressiveness. Administered antipsychotics should be reevaluated at short intervals with the purpose of discontinuation as soon as feasible.
Roaming, delusions, and hallucinations may respond to antipsychotic medications.
Although reports have suggested potential benefits with certain pharmacotherapy on management of FTD in general, evidence from randomized controlled trials is limited [Freedman 2007]. All of the following findings require confirmation with larger clinical trials:
- One double-blind placebo-controlled crossover trial suggests that trazodone, a serotonergic agent, may be beneficial in treating the symptoms of irritability, agitation, depression, and eating disorders in FTD [Lebert et al 2004].
- While an open-label study suggested some benefits on behavioral symptoms with paroxetine, a double-blind placebo-controlled trial of ten subjects found worsening of performance on paired associates learning, reversal learning, and delayed pattern recognition [Moretti et al 2003, Deakin et al 2004].
- A study of galantamine in bvFTD and primary progressive aphasia (PPA) found significant benefits in subjects with PPA but not in those with bvFTD [Kertesz et al 2005]. A follow-up study of 36 individuals who were on galantamine therapy for 18 weeks revealed stabilization but not improvement on language scores in the PPA group [Kertesz et al 2008].
- A 12-month open-label rivastigmine trial showed improvement of behavioral symptoms and decreased caregiver burden in individuals with FTD; however, the treatment did not prevent cognitive decline [Moretti et al 2004].
- A double-blind placebo-controlled crossover study of methylphenidate found attenuation of risk-taking behavior but worsening of spatial span [Rahman et al 2006].
- A small clinical trial of dextroamphetamine treatment on eight individuals with bvFTD revealed improvement of behavioral symptoms [Huey et al 2008].
- A few open-label studies of memantine, a partial NMDA agonist, demonstrated an improvement on the frontal battery inventory in individuals with bvFTD after a six-month trial, but a decline in other cognitive performance [Diehl-Schmid et al 2008]. Among the three subtypes of FTD, PPA-PNFA remained stable on cognitive and functional measurements when treated with memantine [Boxer et al 2009]. A study using [18F]-fluorodeoxyglucose positron emission tomography (FDG-PET) as a surrogate outcome in individuals with semantic dementia found that cortical metabolic activity in salience network hubs was sustained when treated with memantine over a six-month period [Chow et al 2013]. While a meta-analysis suggest some benefit with memantine, the sample sizes were small and further studies with larger samples sizes are needed [Kishi et al 2015].
Note: Donepezil treatment has been associated with exacerbation of disinhibition and compulsion symptoms [Mendez et al 2007].
Surveillance
Patients are often followed in a memory disorder clinic or a similar multidisciplinary clinic involving neurologic and psychiatric services and follow-up medical care.
Evaluation of Relatives at Risk
See Genetic Counseling for issues related to testing of at-risk relatives for genetic counseling purposes.
Therapies Under Investigation
Search ClinicalTrials.gov in the US and EU Clinical Trials Register in Europe for access to information on clinical studies for a wide range of diseases and conditions. Note: There may not be clinical trials for this disorder.