Retinitis Pigmentosa
A number sign (#) is used with this entry because of the extensive genetic heterogeneity of nonsyndromic retinitis pigmentosa as well as the occurrence of retinitis pigmentosa with many generalized disorders.
See INHERITANCE for a list of numbered and unnumbered forms of RP.
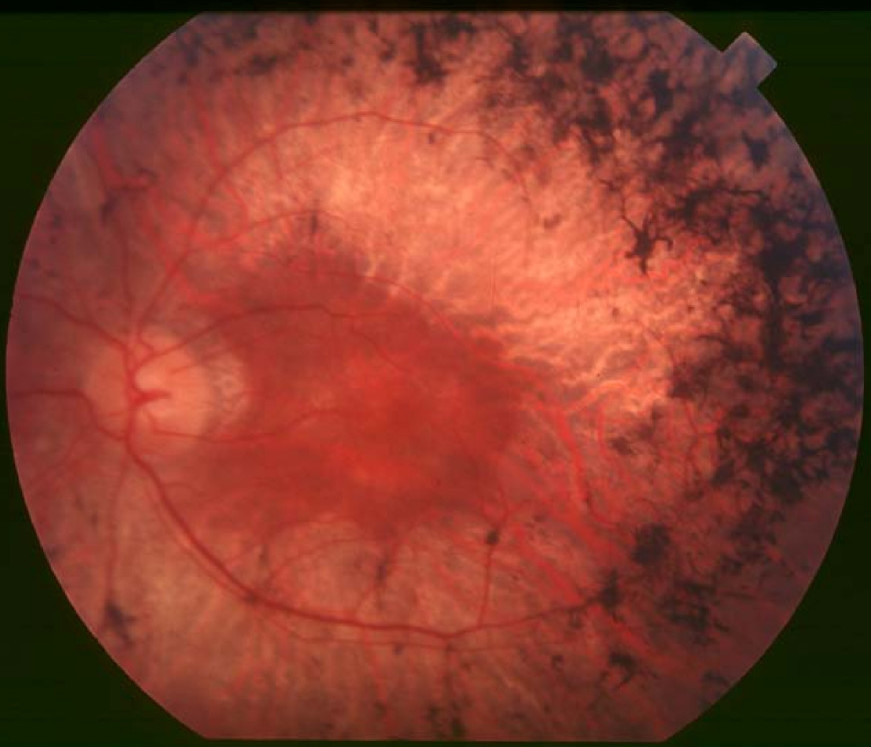
DescriptionRetinitis pigmentosa (RP) refers to a heterogeneous group of inherited ocular diseases that result in a progressive retinal degeneration affecting 1 in 3,000 to 5,000 people (Veltel et al., 2008). Symptoms include night blindness, the development of tunnel vision, and slowly progressive decreased central vision starting at approximately 20 years of age. Upon examination, patients have decreased visual acuity, constricted visual fields, dyschromatopsia (tritanopic; see 190900), and the classic fundus appearance with dark pigmentary clumps in the midperiphery and perivenous areas ('bone spicules'), attenuated retinal vessels, cystoid macular edema, fine pigmented vitreous cells, and waxy optic disc pallor. RP is associated with posterior subcapsular cataracts in 39 to 72% of patients, high myopia, astigmatism, keratoconus, and mild hearing loss in 30% of patients (excluding patients with Usher syndrome; see 276900). Fifty percent of female carriers of X-linked RP have a golden reflex in the posterior pole (summary by Kaiser et al., 2004).
Juvenile Retinitis Pigmentosa
Autosomal recessive childhood-onset severe retinal dystrophy is a heterogeneous group of disorders affecting rod and cone photoreceptors simultaneously. The most severe cases are termed Leber congenital amaurosis (see 204000), whereas the less aggressive forms are usually considered juvenile retinitis pigmentosa (Gu et al., 1997).
Autosomal recessive forms of juvenile retinitis pigmentosa can be caused by mutation in the SPATA7 (609868), LRAT (604863), and TULP1 (602280) genes (see LCA3, 604232, LCA14, 613341, and LCA15, 613843, respectively).
An autosomal dominant form of juvenile retinitis pigmentosa (see 604393) is caused by mutation in the AIPL1 gene (604392).
InheritanceAutosomal Recessive Inheritance
Hartong et al. (2006) cited studies concluding that 50 to 60% of retinitis pigmentosa is inherited as an autosomal recessive. RP1 (180100) can result from homozygous mutation in the RP1 gene (603937.0006); RP4 (613731), from mutation in the rhodopsin gene (RHO; 180380.0023); RP12 (600105), from mutation in CRB1 (604210.0001); RP14 (600132), in TULP1 (602280.0001); RP19 (601718), in ABCA4 (601691.0008); RP20 (613794), in RPE65 (180069.0003); RP25 (602772), in EYS (612424.0001); RP26 (608380), in CERKL (608381.0001); RP28 (606068), in FAM161A (613596); RP35 (610282), in SEMA4A (607292); RP36 (610599), in PRCD (610598.0001); RP37 (611131), in NR2E3 (604485.0007); RP38 (613862), in MERTK (604705.0001); RP39 (613809), in USH2A (608400.0006); RP40 (613801), in PDE6B (180072.0001); RP41 (612095), in PROM1 (604365.0001); RP43 (613810), in PDE6A (180071.0001); RP44 (613769), in RGR (600342.0001); RP45 (613767), in CNGB1 (600724.0001); RP46 (612572), in IDH3B (604526.0001); RP47 (613758), in SAG (181031.0001); RP49 (613756), in CNGA1 (123825.0001); RP51 (613464), in TTC8 (608132.0005); RP53 (612712), in RDH12 (608830); RP54 (613428), in C2ORF71 (613425.0001); RP55 (613575), in ARL6 (608845); RP56 (613581), in IMPG2 (607056); RP57 (613582), in PDE6G (180073); RP58 (613617), in ZNF513 (613598); RP59 (613861), in DHDDS (608172); RP61 (614180), in CLRN1 (606397); RP62 (614181), in MAK (154235); RP64 (614500), in C8ORF37 (614477); RP65 (see 613660), in CDHR1 (609502); RP66 (615233), in RBP3 (180290); RP67 (615565), in NEK2 (604043); RP68 (615725), in SLC7A14 (615720); RP69 (615780), in KIZ (615757); RP71 (616394), in IFT172 (607386); RP72 (616469), in ZNF408 (616454); RP73 (616544), in HGSNAT (610453); RP74 (616562), in BBS2 (606151); RP75 (617023), in AGBL5 (615900); RP76 (617123), in POMGT1 (606822); RP77 (617304), in REEP6 (609346); RP78 (617433), in ARHGEF18 (616432); RP79 (617460), in HK1 (142600); RP80 (617781), in IFT140 (614620); RP81 (617871), in IFT43 (614068); RP82 (615434), in ARL2BP (615407); RP84 (612880) in DHX38 (605584); and RP85 (618345) in AHR (600253).
Loci have been mapped to 16p12 (RP22; 602594), 4q32-q34 (RP29; 612165), and 1p21.1-p13.3 (RP32; 609913) in consanguineous families.
Autosomal Dominant Inheritance
Hartong et al. (2006) stated that about 30 to 40% of retinitis pigmentosa cases show autosomal dominant inheritance. RP1 (180100) results from mutation in the RP1 gene (603937); RP4 (613731), in the rhodopsin gene (RHO; 180380); RP7 (608133), in the peripherin-2 gene (PRPH2; 179605); RP9 (180104), in the gene designated RP9 (607331); RP10 (180105), in IMPDH1 (146690); RP11 (600138), in PRPF31 (606419); RP13 (600059), in PRPF8 (607300); RP17 (600852), in CA4 (114760); RP18 (601414) in PRPF3 (607301); RP19 (601718), in ABCA4 (601691); RP27 (613750), in NRL (162080); RP30 (607921) in FSCN2 (607643); RP31 (609923), in TOPORS (609507); RP33 (610359), in SNRNP200 (601664); RP35 (610282), in SEMA4A (607292.0003); RP37 (611131), in NR2E3 (604485.0006); RP42 (612943), in KLHL7 (611119); RP44 (613769), in RGR (600342.0002); RP48 (613827), in GUCA1B (602275.0001); RP50 (613194), in BEST1 (607854); RP53 (see 612712), in RDH12 (608830); RP60 (613983), in PRPF6 (613979); RP70 (615922), in PRPF4 (607795); and RP83 (618173) in ARL3 (604695).
The RP63 locus (614494) maps to 6q23.
An autosomal dominant disorder described as pericentral retinitis pigmentosa is discussed in 180210.
X-Linked Inheritance
According to Hartong et al. (2006), 5 to 15% of retinitis pigmentosa is inherited through X linkage.
RP2 (312600) is caused by mutation in the RP2 gene (300757). RP23 (300424) is caused by mutation in the OFD1 gene (300170).
A form of X-linked retinitis pigmentosa (XLRP) that had been designated both RP3 and RP15 (300029) is caused by mutation in the RPGR gene (312610). Inheritance of RP3 was described as X-linked recessive, while in RP15, affected males and carrier females presented with early cone involvement, which differs from the typical rod-predominant manifestation of X-linked retinitis pigmentosa. Based on their findings, Vervoort et al. (2000) suggested that mutations in RPGR account for the disease in over 70% of XLRP patients and an estimated 11% of all retinitis pigmentosa patients. Mutations in the RPGR gene can also cause a syndromic form of retinitis pigmentosa (see 300455) as well as other eye phenotypes.
RP6 (312612) has been mapped to chromosome Xp21.3-p21.2; RP24 (300155), to Xq26-q27; and RP34 (300605), to Xq28.
Y-Linked Inheritance
A family showing possible Y-linked inheritance has been reported (RPY; 400004).
Other Forms
RP7 (608133) can be caused by digenic mutations (double heterozygosity) in the PRPH2 gene (179605) and the ROM1 gene (180721).
A syndromic mitochondrial form, which had formerly been called RP8 and RP21, exists (500004), caused by mutation in MTTS2 (590085).
The symbol RP5 has alternately represented a consanguineous Spanish family with a deletion in chromosome 6q (see RP25, 602772), and a family presumed unlinked to rhodopsin in which a RHO mutation was later found (see 613731).
The symbol RP16 had been used to refer to a subset of Sardinian families with autosomal recessive inheritance showing linkage to chromosome 14q11 (Bruford et al., 1994; Wright et al., 1995; Roepman et al., 2000), but was later withdrawn.
Atypical retinitis pigmentosa is observed in a number of other conditions, including the recessive disorders of abetalipoproteinemia (200100), Alstrom syndrome (203800), Refsum syndrome (266500), Bardet-Biedl syndrome (209900), Laurence-Moon syndrome (245800), Usher syndrome (276900), Cockayne syndrome (216400), and pallidal degeneration (260200).
Clinical FeaturesRetinitis pigmentosa is characterized by constriction of the visual fields, night blindness, and fundus changes, including 'bone corpuscle' lumps of pigment. Many cases in successive generations have been reported, e.g., Ayres (1886) 4 generations, Bordley (1908) 5 generations, Allan and Herndon (1944) 5 generations, Heuscher-Isler et al. (1949) 11 cases in 3 generations, and Rehsteiner (1949) 16 cases in 4 generations. The most extensively affected family reported is probably that studied by Beckershaus (1925). Sunga and Sloan (1967), who described a family with 13 affected in 3 generations, including 2 instances of male-to-male transmission, remarked on the wide variability in the rate of visual deterioration among individuals of the same family. The pathophysiology of retinitis pigmentosa was discussed by Dowling (1966), who presented experiments suggesting that exposure to bright light may accelerate the degenerative process.
In a survey of retinitis pigmentosa in 5 Swiss cantons, Ammann et al. (1961) found congenital deafness associated in 16 of 118 living cases (see 276900).
Kaplan et al. (1990) reviewed 93 cases of retinitis pigmentosa. Sporadic cases represented the major category (42%). In this group, at least 3 clinical forms could be recognized: cone-rod dystrophy, early-onset severe forms, and late-onset moderate forms. At the beginning of the disease, the hereditary nature of the sporadic forms was difficult to ascertain, especially between 7 and 10 years of age, and only the clinical course could possibly provide information regarding the mode of inheritance. A high level of consanguinity and a preponderance of males in the early-onset, severe sporadic forms (including cone-rod dystrophy) suggested autosomal or X-linked recessive inheritance, while increased paternal age in late-onset forms was suggestive of autosomal dominant mutations.
Ben-Arie-Weintrob et al. (2005) reviewed the published histopathologic findings of patients with retinitis pigmentosa or an allied disease in whom the responsible gene defect had been identified.
Janaky et al. (2007) analyzed multifocal electroretinograms (mfERGs) in patients with RP with various forms of inheritance and duration of disease, with constricted visual fields and visual acuity satisfactory for steady fixation. Their results suggested highly variable central responses and groups of cones with preserved function in areas previously considered nonresponsive. The authors noted that the high variability of the central responses could have been the result of variable foveal cone density, with differences in inheritance- and duration-related cone degeneration at the time of examination. Janaky et al. (2007) stressed the value of step-by-step analysis of the trace array of the mfERGs, which could reveal the groups of cones that were still functioning.
Macrae (1982) tabulated the percentage frequency of the 3 mendelian forms of retinitis pigmentosa, as observed in 5 studies including his own in Ontario. Autosomal dominants varied from 9% (in Switzerland) to 39% (in the U.K.); autosomal recessives from 90% (in Switzerland) to 15% (in the U.K.) and X-linked from 1% (in Switzerland and Russia) to 15% (in the U.K.). In the City of Birmingham, England, Bundey and Crews (1984) found a prevalence of retinitis pigmentosa for all ages of 1 in about 5,000.
By clinical, electrophysiologic, and psychophysical criteria, Fishman et al. (1985) discerned 4 types of autosomal dominant RP among 84 patients. Type 1 showed diffuse fundus pigmentary changes and nondetectable cone and rod functions by electroretinogram (ERG). Types 2 and 3 showed more apparent pigmentary changes in the inferior retina. Type 2 showed marked loss of rod ERG function with prolonged cone implicit times, whereas type 3 patients showed substantial rod function and normal cone implicit times. Type 4 had funduscopically and functionally 'delimited' disease.
Galbraith et al. (1986) studied 34 patients with RP: 23 sporadic, 3 autosomal dominant, 7 autosomal recessive, and 1 X-linked. Antibodies reactive with heterologous neural tissue were found in 17 of the 34, in 1 of 30 normal controls, and also in disease-free first-degree relatives and spouses of RP patients. The antibodies were specific for high molecular weight protein subunits of neurofilaments. These workers thought that release of piled-up neurofilaments from damaged neurons in RP triggers B lymphocytes autoreactive to neurofilament antigens.
In Norway, Grondahl (1987) found retinitis pigmentosa in 101 persons in 53 families. The prognosis for visual function was most favorable for the autosomal dominant group (38 patients from 8 families). The autosomal recessive group (40 patients from 25 families) and the 19 solitary cases were heterogeneous, with prognosis ranging from favorable to very bad. Intrafamilial correlation was higher in the autosomal recessive group than in the autosomal dominant group. The overall prevalence of RP in Norway was 1/4,440, the autosomal dominant form being the most frequent. Atypical RP occurs in a number of other conditions, the Flynn-Aird syndrome (136300) being an autosomal dominant example.
A clinically distinct variant referred to as type II ADRP was found to segregate independently of chromosome 3q markers (Inglehearn et al., 1990; Farrar et al., 1990; Blanton et al. (1990, 1991)). Field et al. (1982) had presented data that excluded an autosomal dominant RP gene from nearly 40 cM around the transferrin (TF; 190000) locus, at 3q21. In 2 families with late-onset, moderately severe RP, Kaplan et al. (1990) excluded linkage to a marker close to rhodopsin (180380). Massof and Finkelstein (1981) had suggested that autosomal dominant retinitis pigmentosa can be divided into type I (early onset) with night blindness before 10 years, and type II (late onset) beginning in the third decade. These 2 types can be further distinguished on the basis of persistence of a measurable rod electroretinogram (Arden et al., 1983) and also on the distribution of pigmentation in the affected retina in the early stages of the disease (Lyness et al., 1985). Classification on the latter basis gives the diffuse (D) and regional (R) types, which correspond to the early (type I) and late (type II) onset categories of Massof and Finkelstein (1981), respectively. Blanton et al. (1991) commented, however, that there was 'no remarkable clinical disparity in the expression of disease caused by the different loci' identified by linkage studies.
Charles Bonnet Syndrome
Through a telephone interview of 72 patients with severe vision impairment (acuity less than 20/200 in the better eye and/or visual field restriction to less 10 degrees), who were part of a larger natural history study of RP in Australia, O'Hare et al. (2015) identified 27 (37.5%) with recurrent visual hallucinations consistent with Charles Bonnet syndrome (CBS). Of the 27 patients, 13 experienced simple hallucinations comprising inanimate light patterns that lasted several seconds, and 10 reported that episodes manifested at random, with no noted temporal patterns or known triggers. Of the 17 patients who were aware of the exacerbating circumstances, 11 experienced hallucinations when tired and 3 when concentrating on an activity or task. Twenty-one patients reported that the hallucinations disappeared on their own, whereas 6 reported that episodes ceased upon intentionally closing their eyes. Eighteen patients reported emotional distress from their experience of visual hallucinations. O'Hare et al. (2015) stressed the importance of diagnosis and active management of CBS during routine ophthalmologic care and clinical treatment trials.
PathogenesisBird (1995) reviewed literature concerning photoreceptor dystrophies and assessed their potential impact on concepts of pathogenesis of disease and clinical practice.
Clarke et al. (2000) studied the kinetics of neuronal death in 12 models of photoreceptor degeneration, hippocampal neurons undergoing excitotoxic cell death, a mouse model of cerebellar degeneration, and in Parkinson (168600) and Huntington (143100) diseases. In all models the kinetics of neuronal death were exponential and better explained by mathematical models in which the risk of cell death remains constant or decreases exponentially with age. These kinetics argue against the cumulative damage hypothesis; instead, the time of death in any neuron is random. Clarke et al. (2000) argued that their findings are most simply accommodated by a '1-hit' biochemical model in which mutation imposes a mutant steady state on the neuron and a single event randomly initiates cell death. This model appears to be common to many forms of neurodegeneration and has implications for therapeutic strategies in that the likelihood that a mutant neuron can be rescued by treatment is not diminished by age, and therefore treatment at any stage of illness is likely to confer benefit.
Population GeneticsSharon and Banin (2015) stated that the reported prevalence of nonsyndromic RP in American and European populations is approximately 1:5,260 on average. Sharon and Banin (2015) found a prevalence of 1:2,086 in the Jerusalem region. The prevalence was higher in Arab Muslims (1:1,798) compared to Jews (1:2,230). In their cohort of 183 different families, 49% had autosomal recessive inheritance. The genetic cause of RP was determined in 64 (35%) of the families; in 42 (66%) of the 64 families, the cause was a founder mutation.
Molecular GeneticsFor a discussion of the molecular genetics of particular forms of retinitis pigmentosa, see the pertinent entries, listed in the INHERITANCE section.
Sohocki et al. (2001) screened for mutations in 5 genes in a large number of individuals with retinitis pigmentosa and other inherited retinopathies. In the retinitis pigmentosa group there were 423 tested individuals, of which 206 had autosomal dominant RP, 138 had isolated/recessive RP, and 79 had RP of unknown nature because of unavailability of family history. Mutations in the rhodopsin gene (180380) were found in 59 of the 423 patients. Nineteen had mutations in the peripherin/RDS gene. Eight had mutations in the RP1 gene (603937). Two had mutations in the CRX gene (602225), and none had mutations in the AIPL1 gene (604392), which has been found to be mutant in cases of Leber optic atrophy and in retinal disorders with cone involvement.
Kondo et al. (2004) used an established strategy of flexible, multiplexed, microsatellite-based homozygosity mapping to identify mutations in known candidate genes in 59 patients with autosomal recessive or simplex retinitis pigmentosa. Of the 59 probands examined (12 consanguineous and 47 nonconsanguineous), 24 had a mean of 1.4 genes showing homozygosity for all markers within the corresponding gene region. Subsequent direct sequencing revealed 3 homozygous mutations. Two of them were novel mutations in the genes TULP1 (602280.0006) and CNGB1 (600724.0002). The other was a mutation in the RPE65 gene (180069.0008) that was known to cause Leber congenital amaurosis (204000). The clinical features of each patient, together with the cosegregation analysis, strongly supported the pathogenicity of these mutations.
Coppieters et al. (2007) noted RetNet (the Retinal Information Network) as recording 17 autosomal dominant loci, 25 autosomal recessive loci, and 6 X-linked recessive loci causing retinitis pigmentosa. Autosomal dominant retinitis pigmentosa (adRP) represents a genetically heterogeneous group of retinal dystrophies in which 54% of all cases can be attributed to 17 disease loci.
DiagnosisKondo et al. (2003) described a hierarchical approach for efficient genetic diagnosis of autosomal dominant retinitis pigmentosa.
Clinical ManagementSwanson et al. (2000) studied the effect of stimulus size on sensitivity of patients with retinitis pigmentosa as measured by automated static perimetry. For decades, the standard for documenting visual field loss in RP patients had been manual kinetic (Goldmann) perimetry. It has gradually been replaced by automated static perimetry, the standard for assessing glaucomatous visual field loss. Swanson et al. (2000) performed automated static perimetry on their RP patients using stimulus sizes III (0.43 degrees diameter) and V (1.72 degrees diameter). They cautioned that in damaged regions of the visual fields of RP patients, an increase in stimulus size from III to V could produce abnormally large increases in perimetric sensitivity. They concluded that size III may be more useful than size V for detection of field abnormality, whereas size V may be more useful than size III for observing progression of advanced RP.
Berger et al. (2003) published a 1-year follow-up on 8 patients who underwent adult human photoreceptor transplantation as treatment for advanced RP. They concluded that allogeneic adult human photoreceptor transplantation is feasible in RP but was not associated with rescue of central vision or a delay in visual loss in their patients. However, they stated that any possible slowing in the rate of retinal degeneration would take many years to determine.
The Royal College of Surgeons (RCS) rat is a widely studied, classic model of recessively inherited retinal degeneration in which the retinal pigment epithelium (RPE) fails to phagocytose shed outer segments, and photoreceptor cells subsequently die. Lawrence et al. (2004) found that engineered Schwann cells sustained retinal structure and function in the dystrophic RCS rat. Cells overexpressing glial cell line-derived neurotrophic factor (GDNF; 600837) or brain-derived neurotrophic factor (BDNF; 113505) had a greater effect on photoreceptor survival than the parent line or sham surgery. The authors concluded that their study demonstrated that ex vivo gene therapy and subsequent cell transplantation could be effective in preserving photoreceptors from the cell death that normally accompanies retinal degeneration.
Busskamp et al. (2010) demonstrated that expression of archaebacterial halorhodopsin in light-insensitive cones can substitute for the native phototransduction cascade and restore light sensitivity in mouse models of retinitis pigmentosa. Resensitized photoreceptors activate all retinal cone pathways, drive sophisticated retinal circuit functions (including directional selectivity), activate cortical circuits, and mediate virtually guided behaviors. Using human ex vivo retinas, Busskamp et al. (2010) showed that halorhodopsin can reactivate light-insensitive human photoreceptors. Finally, Busskamp et al. (2010) identified blind patients with persisting, light-insensitive cones for potential halorhodopsin-based therapy.
NomenclatureInglehearn and Hardcastle (1996) referred to the confused state of the various forms of RP to which numbers had been assigned. Because of the tendency to assign locus numbers while data were still only tentative, RP1 (180100) moved from chromosome 1 to chromosome 8, RP5 (see 613731) no longer exists, and RP8 (see 500004) was never a defined locus but merely a family unlinked to the previous 7 loci. RP4 (613731) (due to rhodopsin mutations) and RP7 (608133) (due to peripherin/RDS (179605) mutations) are more helpfully referred to simply by the gene names. Inglehearn and Hardcastle (1996) provided in their Table 1 a comprehensive listing of locus nomenclature for human inherited retinal degenerations.
HeterogeneityGal et al. (1990) found evidence of close linkage with no recombination (maximum lod = 4.08 at theta = 0.00) with RBP1 (180260), but looser linkage with several recombinants with RHO and its closely linked marker, D3S47.
In a large South African family of British origin, Greenberg et al. (1992) failed to find linkage between autosomal dominant RP and D3S47; linkage was excluded at a recombination fraction of almost 0.10.
Animal ModelNilsson et al. (2001) studied retinal blood flow during the course of autosomal recessive progressive retinal atrophy (PRA) in Abyssinian cats. Both the clinical course and electrophysiologic findings of PRA greatly resemble those found in patients with retinitis pigmentosa. They found that retinal blood flow decreased significantly and iridal resistance to blood flow increased significantly at a late stage of retinal degeneration, while indomethacin had no effect on the iris circulation in normal cats. The retinal formation of lactate was significantly lower in cats with PRA than in normal cats, while the uptake of glucose was not significantly different in cats with PRA. They concluded that increased vascular resistance in the iris was caused at least in part by cyclooxygenase products because iris blood flow more than doubled after treating the cats with indomethacin.
HistoryThe striking pedigree by Franceschetti (1953) was reproduced in the book by Francois (1961).
Babel (1972) suggested that heterozygotes of retinitis pigmentosa develop fundus changes typical of the homozygote after measles.
Boughman et al. (1980) estimated the overall frequency at about 1 in 3,700, whereas the incidence of the recessive type, with at least 2 genocopies, was estimated to be about 1 in 4,450. No evidence of ethnic heterogeneity was found.
Heckenlively et al. (1981) identified 43 cases of autosomal recessive RP among the Navajo Indians. Heckenlively (1982) stated that he had seen only 1 person with Indian blood who had the fundus appearance of the Navajo RP, which may be a distinct entity: signs of night blindness were noted by parents as early as age 2 years. In the early stages, the fundus was characterized by a gray granular appearance in areas of focal thinning of retinal pigment epithelium, exposing the choroid. As the disorder progressed, these areas became confluent and islands of intact retinal pigment epithelium were noted. There was minimal pigment aggregation or dispersion, and bone spicules or large clumps of pigment were not seen.
In Shanghai, Hu (1982) analyzed 151 pedigrees with 209 cases of RP. Of these cases, the proportions of autosomal recessive (AR), autosomal dominant (AD), X-linked recessive (XR), and simplex cases were 33.1, 11, 7.7 and 48.3%, respectively. In the AD, AR and XR types, the average ages of onset were 24.7, 22.9, and 5 years, respectively. The average refractive errors in the AD, AR, and XR types were -1.88, -2.37 and -5.72 D, respectively. The gene frequency calculated from frequency of parental consanguinity was much less than that calculated from the frequency of AR (plus simplex cases). Possibly the existence of many different forms of AR RP was the explanation. The number of different mutations causing RP was estimated to lie between 11 and 41.