Col1a1/2 Osteogenesis Imperfecta
Summary
Clinical characteristics.
COL1A1/2 osteogenesis imperfecta (COL1A1/2-OI) is characterized by fractures with minimal or absent trauma, variable dentinogenesis imperfecta (DI), and, in adult years, hearing loss. The clinical features of COL1A1/2-OI represent a continuum ranging from perinatal lethality to individuals with severe skeletal deformities, mobility impairments, and very short stature to nearly asymptomatic individuals with a mild predisposition to fractures, normal dentition, normal stature, and normal life span. Fractures can occur in any bone but are most common in the extremities. DI is characterized by gray or brown teeth that may appear translucent, wear down, and break easily. COL1A1/2-OI has been classified into four types based on clinical presentation and radiographic findings. This classification system can be helpful in providing information about prognosis and management for a given individual. The four more common OI types are now referred to as follows:
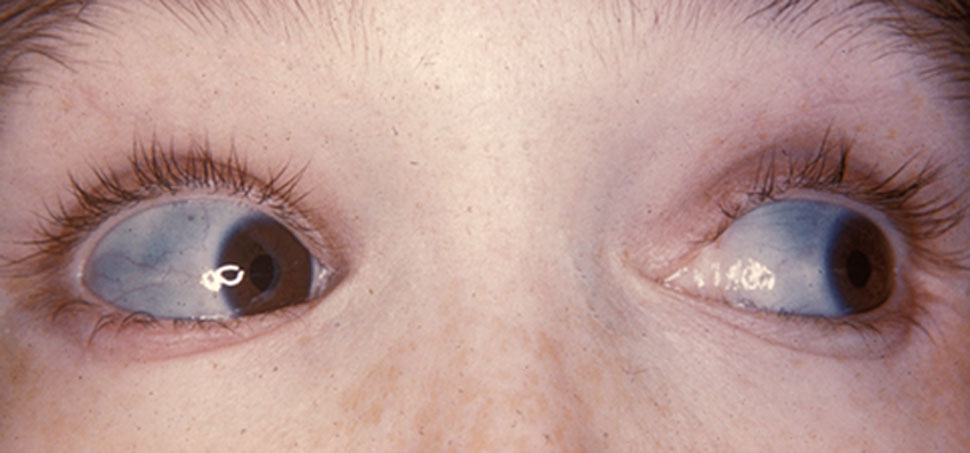
- Classic non-deforming OI with blue sclerae (previously OI type I)
- Perinatally lethal OI (previously OI type II)
- Progressively deforming OI (previously OI type III)
- Common variable OI with normal sclerae (previously OI type IV)
Diagnosis/testing.
The diagnosis of COL1A1/2-OI is established in a proband by identification of a heterozygous pathogenic or likely pathogenic variant in COL1A1 or COL1A2 by molecular genetic testing.
Management.
Treatment of manifestations: Ideally, management is by a multidisciplinary team including specialists in medical management of OI, clinical genetics, orthopedics, rehabilitation medicine, pediatric dentistry, otology/otolaryngology, and mental health. Parents / other caregivers must practice safe handling techniques. Mainstays of treatment include: bracing of limbs depending on OI severity; orthotics to stabilize lax joints; physical activity; physical and occupational therapy to maximize bone stability, improve mobility, prevent contractures, prevent head and spine deformity, and improve muscle strengthening; mobility devices as needed; and pain management. Fractures are treated with: as short a period of immobility as is practical; small and lightweight casts; physical therapy as soon as casts are removed; and intramedullary rodding when indicated to provide anatomic positioning of limbs. Progressive scoliosis in severe OI may not respond well to conservative or surgical management. Bisphosphonates continue to be used most extensively in severely affected children with OI. Surgical treatment for basilar impression should be done in a center experienced in the necessary procedures. Dental care strives to maintain both primary and permanent dentition, a functional bite or occlusion, optimal gingival health, and overall appearance. Conductive hearing loss may be improved with middle ear surgery; later-onset sensorineural hearing loss is treated in the same manner as when caused by other conditions. Mental health support through psychiatry/psychology and appropriate social worker intervention can improve quality of life.
Prevention of secondary complications: During general anesthesia, proper positioning on the operating room table and use of cushioning such as egg crate foam can help avoid fractures.
Surveillance: Orthopedic evaluation with ancillary therapy services (physical and rehabilitation medicine) as indicated every three months until age one year, every six months from ages one to three years, and then annually or with any new fractures. Physical therapy evaluation in infancy for those with motor delays and as needed to improve mobility and function. CT and/or MRI examination with views across the base of the skull to evaluate for basilar impression if concerning signs or symptoms are present. Cervical spine flexion and extension radiographs in children able to cooperate with the examination or before participating in sporting activities in more mildly affected individuals. Twice-yearly dental visits beginning in early childhood or even infancy for those with (or at risk for) DI. Hearing evaluation at three- to five-year intervals from age five years until hearing loss is identified, then as indicated based on the nature and degree of hearing loss and associated interventions.
Agents/circumstances to be avoided: Contact sports should be avoided.
Genetic counseling.
COL1A1/2-OI is inherited in an autosomal dominant manner. The proportion of affected individuals who represent simplex cases (i.e., a single occurrence of the disorder in a family) varies by the severity of disease. Approximately 60% of probands with mild OI represent simplex cases. Virtually 100% of probands with progressively deforming or perinatally lethal OI represent simplex cases and have a de novo pathogenic variant or a pathogenic variant inherited from a parent with somatic and/or germline mosaicism. Parental somatic and/or germline mosaicism is present in up to 16% of families. Each child of an individual with a dominantly inherited form of COL1A1/2-OI has a 50% chance of inheriting the causative variant and of developing some manifestations of OI. Prenatal testing in at-risk pregnancies can be performed by molecular genetic testing if the COL1A1 or COL1A2 causative variant has been identified in an affected relative. Ultrasound examination performed in a center with experience in diagnosing OI can be valuable in the prenatal diagnosis of the lethal form and most severe forms prior to 20 weeks' gestation; milder forms may be detected later in pregnancy if fractures or deformities occur.
Diagnosis
An algorithm for the diagnosis of osteogenesis imperfecta (OI) has been published [Basel & Steiner 2009]. See Figure 1.

Figure 1.
Recommended testing algorithm for evaluation of osteogenesis imperfecta Adapted from Basel & Steiner [2009]
Suggestive Findings
COL1A1/2 osteogenesis imperfecta (OI) should be suspected in individuals with the following clinical, radiographic, and laboratory features.
Clinical features (Table 1)
- Fractures with minimal or no trauma in the absence of other factors, such as non-accidental trauma (NAT) or other known disorders of bone
- Short stature or stature shorter than predicted based on stature of unaffected family members, often with bone deformity
- Blue/gray scleral hue
- Dentinogenesis imperfecta (DI)
- Progressive, postpubertal hearing loss
- Ligamentous laxity and other signs of connective tissue abnormality
- Family history of OI, usually consistent with autosomal dominant inheritance
Table 1.
Clinical Features of COL1A1/2 Osteogenesis Imperfecta by Type
| Type | MOI | Severity | Fractures | Bone Deformity | Stature | DI | Sclerae | Hearing Loss |
|---|---|---|---|---|---|---|---|---|
| Classic non-deforming OI w/blue sclerae | AD | Mild | Few to 100 | Uncommon | Normal or slightly short for family | Rare | Blue | Present in ~50% |
| Perinatally lethal OI | AD | Perinatal lethal | Multiple fracture of ribs, minimal calvarial mineralization, platyspondyly, marked compression of long bones | Severe | Severely short | + | Dark blue | — |
| Progressively deforming OI | AD | Severe | Thin ribs, platyspondyly, thin gracile bones w/many fractures, "popcorn" epiphyses common | Moderate to severe | Very short | + | Blue | Frequent |
| Common variable OI w/normal sclerae | AD | Moderate to mild | Multiple | Mild to moderate | Variably short | +/– | Normal to gray | Some |
AD = autosomal dominant; DI = dentinogenesis imperfecta; MOI = mode of inheritance
Radiographic features of OI change with age. The major findings include the following (Table 2):
- Fractures of varying ages and stages of healing, often of the long bones but may also rarely involve ribs and skull. Metaphyseal fractures can be seen in a very small number of children with OI. Rib fractures are much more common in NAT than in OI.
- "Codfish" vertebrae, which are the consequence of spinal compression fractures, seen more commonly in adults
- Wormian bones, defined as "sutural bones which are 6 mm by 4 mm (in diameter) or larger, in excess of ten in number, with a tendency to arrangement in a mosaic pattern" [Cremin et al 1982]. Wormian bones are suggestive of but not pathognomonic for OI.
- Protrusio acetabuli, in which the socket of the hip joint is too deep and the acetabulum bulges into the cavity of the pelvis causing intrapelvic protrusion of the acetabulum
- Low bone mass or osteoporosis detected by dual energy x-ray absorptiometry (DEXA). Bone density can be normal, especially in individuals with OI type I, as DEXA measures mineral content rather than collagen [Deodhar & Woolf 1994, Paterson & Mole 1994, Cepollaro et al 1999, Lund et al 1999].Note: (1) A major determinant of bone density may be the individual's ability to ambulate. (2) Bone density standards for children under age two years have been determined after sampling very small populations (often <10 persons); thus, reliability is an issue. (3) Bone density standards for children are based on height; corrections for short stature of severely affected individuals need to be made. (4) Bone density is not typically measured in children before age four years because of their inability to lie still, though this may be accomplished with patience in sleeping infants. (5) The purpose of measuring bone density in individuals known to have OI is to allow for monitoring of the individual's bone density over time, and not for comparison with unaffected individuals.
Table 2.
Radiographic Findings of COL1A1/2 Osteogenesis Imperfecta by Type
| Type | Severity | Skull | Back | Extremities | Other |
|---|---|---|---|---|---|
| Classic non-deforming OI w/blue sclerae | Mild | Wormian bones | Codfish vertebrae (adults) | Thin cortices | Osteopenia |
| Perinatally lethal OI | Perinatal lethal | Undermineralization; plaques of calcification | Platyspondyly | Severely deformed; broad, crumpled, bent femurs | Small beaded ribs (pathognomonic) |
| Progressively deforming OI | Severe | Wormian bones | Codfish vertebrae; kyphoscoliosis | Flared metaphyses ("popcorn"-like appearance in childhood), bowing, thin cortices | Thin ribs, severe osteoporosis |
| Common variable OI w/normal sclerae | Intermediate | ± wormian bones | Codfish vertebrae | Thin cortices | Protrusio acetabuli in a subset |
Laboratory features
- Serum concentrations of vitamin D, calcium, phosphorous, and alkaline phosphatase are typically normal; however, alkaline phosphatase may be elevated acutely in response to fracture and rare instances of abnormally low alkaline phosphatase levels have been noted anecdotally in severe OI.
- Analysis of type 1 collagen synthesized in vitro by culturing dermal fibroblasts obtained from a small skin biopsy reflects the structure and quantity of the collagen. The sensitivity of biochemical testing is approximately 90% in individuals with clinically confirmed OI [Wenstrup et al 1990; PH Byers, personal communication]. Biochemical analysis is essentially no longer used clinically with the advances in molecular diagnostics.
Establishing the Diagnosis
The diagnosis of COL1A1/2-OI is established in a proband by identification of a heterozygous pathogenic or likely pathogenic variant in COL1A1 or COL1A2 by molecular genetic testing (see Table 3). An approach to the molecular diagnosis of OI has been published (see Figure 2) [van Dijk et al 2012], but such approaches are in flux as technology is changing rapidly.

Figure 2.
Preferred diagnostic flow in OI The approach to diagnosis is designed to maximize the likelihood that causative variants will be identified in all affected individuals or assign those without causative variants to research pools. This flow assumes that (more...)
Molecular genetic testing approaches can include a combination of gene-targeted testing (concurrent gene testing, multigene panel) and comprehensive genomic testing (exome sequencing, genome sequencing) depending on the phenotype.
Option 1
When the phenotypic and laboratory findings suggest the diagnosis of COL1A1/2-OI, molecular genetic testing approaches can include concurrent gene testing or use of a multigene panel:
- Concurrent gene testing. Sequence analysis of COL1A1 and COL1A2 detects small intragenic deletions/insertions and missense, nonsense, and splice site variants; typically, exon or whole-gene deletions/duplications are not detected. Perform sequence analysis first. If no pathogenic variant is found, perform gene-targeted deletion/duplication analysis to detect intragenic deletions or duplications.
- A multigene panel that includes COL1A1, COL1A2, and other genes of interest (see Differential Diagnosis) is most likely to identify the genetic cause of the condition at the most reasonable cost while limiting identification of variants of uncertain significance and variants in genes that do not explain the underlying phenotype. Note: (1) The genes included in the panel and the diagnostic sensitivity of the testing used for each gene vary by laboratory and are likely to change over time. (2) Some multigene panels may include genes not associated with the condition discussed in this GeneReview. (3) In some laboratories, panel options may include a custom laboratory-designed panel and/or custom phenotype-focused exome analysis that includes genes specified by the clinician. (4) Methods used in a panel may include sequence analysis, deletion/duplication analysis, and/or other non-sequencing-based tests. For this disorder, a multigene panel that also includes deletion/duplication analysis is recommended (see Table 3).For an introduction to multigene panels click here. More detailed information for clinicians ordering genetic tests can be found here.
Option 2
When the phenotype is indistinguishable from many other inherited disorders characterized by bone fragility and/or skeletal dysplasia, comprehensive genomic testing (which does not require the clinician to determine which gene[s] are likely involved) is the best option. Exome sequencing is most commonly used; genome sequencing is also possible and becoming more widely available.
For an introduction to comprehensive genomic testing click here. More detailed information for clinicians ordering genomic testing can be found here.
Table 3.
Molecular Genetic Testing Used in COL1A1/2 Osteogenesis Imperfecta
| Gene 1, 2 | Proportion of OI Attributed to Pathogenic Variants in Gene | Proportion of Pathogenic Variants 3 Detectable by Method | |
|---|---|---|---|
| Sequence analysis 4 | Gene-targeted deletion/duplication analysis 5 | ||
| COL1A1 | ~5%-70% 6 | >95% 7 | 1%-2% 8 |
| COL1A2 | ~5%-30 6 | >95% 7 | 1%-2% 8 |
- 1.
Genes are listed in alphabetic order.
- 2.
See Table A. Genes and Databases for chromosome locus and protein.
- 3.
See Molecular Genetics for information on allelic variants detected in this gene.
- 4.
Sequence analysis detects variants that are benign, likely benign, of uncertain significance, likely pathogenic, or pathogenic. Pathogenic variants may include small intragenic deletions/insertions and missense, nonsense, and splice site variants; typically, exon or whole-gene deletions/duplications are not detected. For issues to consider in interpretation of sequence analysis results, click here.
- 5.
Gene-targeted deletion/duplication analysis detects intragenic deletions or duplications. Methods used may include quantitative PCR, long-range PCR, multiplex ligation-dependent probe amplification (MLPA), and a gene-targeted microarray designed to detect single-exon deletions or duplications.
- 6.
PH Byers, personal communication
- 7.
Sequence analysis of COL1A1 and COL1A2 cDNA to detect pathogenic variants in the coding sequence and sequence analysis of COL1A1 and COL1A2 genomic DNA to detect pathogenic variants that alter either sequence or stability of mRNA identify close to 100% of pathogenic variants in these two genes.
- 8.
van Dijk et al [2010] and data derived from Human Gene Mutation Database [Stenson et al 2017]
Clinical Characteristics
Clinical Description
The severity of COL1A1/2 osteogenesis imperfecta (COL1A1/2-OI) ranges from perinatal lethality to individuals with severe skeletal deformities, mobility impairments, and very short stature to nearly asymptomatic individuals with a mild predisposition to fractures, normal stature, and normal life span.
COL1A1/2-OI is classified into four more common types based on clinical presentation, radiographic features, family history, and natural history [Sillence et al 1979]. An update of the Sillence classification has been proposed and has gained some acceptance [Emery & Rimoin 2012]. Although this classification of COL1A1/2-OI into types is helpful in providing information about prognosis and management of a given individual, the features of different types of COL1A1/2-OI overlap and it is not always easy to categorize the extent of the clinical disorder. It is helpful to remember that the severity of clinical and radiographic features lies on a continuum and that the "types" are defined using characteristics that appear to form clinical "nodes." Interfamilial variability is apparent among individuals with the same OI type and intrafamilial variability is apparent among individuals with the same causative variant. Nonetheless, it is reasonable to continue to think of COL1A1/2-OI in terms of these types in order to provide information about the expected natural history of the disorder.
Classic non-deforming OI with blue sclerae (previously OI type I) is characterized by blue sclerae and normal stature. A small proportion of infants with OI type I have femoral bowing at birth. The first fractures may occur at birth or with diapering. More often, the first fractures occur when the infant begins to walk and, more importantly, to fall. Fractures generally occur at a rate of a few to several per year and then decrease in frequency after puberty. Fracture frequency often increases again in adulthood, especially in postmenopausal women and men beyond the fifth decade [Paterson et al 1984]. Affected individuals may have anywhere from a few fractures to more than 100, but the fractures usually heal normally with no resulting deformity.
Most affected individuals have normal or near normal stature but are often shorter than other members of their families and shorter than predicted based on parental heights.
Joint hypermobility predisposes to a number of minor comorbidities. The primary clinical concern is early-onset degenerative joint disease due to malalignment of articular surfaces.
In their classification of OI, Sillence et al [1979] designated a subset of classic non-deforming OI with dentinogenesis imperfecta (DI) (OI type IB). In individuals with DI, morbidity results not from dental decay but rather from premature wearing down of the teeth. DI can be a significant cosmetic concern. Dental eruption in classic non-deforming OI can sometimes occur early.
Progressive hearing loss occurs in about 50% of adults with classic non-deforming OI, beginning as a conductive hearing loss but often with an additional sensorineural hearing loss component in time.
Perinatally lethal OI (previously OI type II). Abnormalities characteristic of perinatally lethal OI are evident at birth. Weight and length are small for gestational age. The sclerae are dark blue and connective tissue is extremely fragile. The skull is large for the body size and soft to palpation. Callus formation on the ribs may be palpable. Extremities are short and bowed. Hips are usually flexed and abducted in a "frog-leg" position. Although some fetuses with perinatally lethal OI die in utero or are spontaneously aborted, more typically infants die in the immediate perinatal period. More than 60% of affected infants die on the first day; 80% die within the first week; survival beyond one year is exceedingly rare and usually involves intensive support such as continuous assisted ventilation [Byers et al 1988]. Death usually results from pulmonary insufficiency related to the small thorax, rib fractures, or flail chest because of unstable ribs. Those who survive the first few days of life may not be able to ingest sufficient calories because of respiratory distress.
Histologic evaluation of bone from infants with perinatally lethal OI shows marked reduction in collagen in secondary trabeculae and cortical bone [Horton et al 1980]. Cortical bone is hypercellular with large osteocytes. Trabeculae contain woven bone with large immature osteoblasts [Cole et al 1992, Cole & Dalgleish 1995].
Progressively deforming OI (previously OI type III). The diagnosis of progressively deforming OI is readily apparent at birth. Fractures in the newborn period, simply with handling of the infant, are common. In some affected infants, the number and severity of rib fractures lead to death from pulmonary failure in the first few weeks or months of life.
Infants who survive this period generally fare well, although most do not walk without assistance and usually use a wheelchair or other assistance for mobility because of severe bone fragility and marked bone deformity. Affected individuals have as many as 200 fractures and progressive deformity even in the absence of obvious fracture. Progressively deforming OI is often difficult to manage orthopedically, even with intramedullary rod placement.
Growth is extremely delayed and adults with progressively deforming OI are among the shortest individuals known, with some having adult stature of less than one meter.
Intellect is normal unless there have been intracerebral hemorrhages (extremely rare). Faqeih et al [2009] published a report identifying increased risk for intracranial hemorrhage (ICH) in a "small number" of individuals who were identified to have pathogenic variants affecting exon 49 of COL1A2, which codes for the most carboxy-terminal part of the triple-helical domain of the collagen alpha-2(I) chain. They concluded that this pathogenic variant appeared to increase the risk for abnormal limb development and intracranial bleeding. Budsamongkol et al [2019] reported a young boy with marked joint hypermobility, significant DI, brachydactyly, and a COL1A2 pathogenic variant found to be associated with ICH by Faqeih et al [2009]. The boy had not experienced an ICH, but as some of the original affected individuals only presented with ICH in their teenage years, this does not eliminate the risk in this young individual.
Even within progressively deforming OI, considerable heterogeneity is observed at the clinical level. Some individuals have normal-appearing teeth and facies while others have DI, a large head, and enlarged ventricles that reflect the soft calvarium. Relative macrocephaly and barrel chest deformity are observed. Usually sclerae are blue in infancy but lighten with age. Hearing loss generally begins in the teenage years. As molecular testing of this subgroup further differentiates those with COL1A1/2-OI from the autosomal recessive forms, the clinical profile of this heterogeneous group will become more refined.
Basilar impression, an abnormality of the craniovertebral junction caused by descent of the skull on the cervical spine, is common. Basilar impression is characterized by invagination of the margins of the foramen magnum upward into the skull, resulting in protrusion of the odontoid process into the foramen magnum. Basilar impression may progress to brain stem compression, obstructive hydrocephalus, or syringomyelia because of direct mechanical blockage of normal CSF flow [Charnas & Marini 1993, Sillence 1994, Hayes et al 1999]. Symptoms of basilar impression become apparent with neck flexion. Findings include posterior skull pain, C2 sensory deficit, tingling in the fourth and fifth digits, and numbness in the medial forearm. When swimming, affected individuals may perceive that water temperature differs below and above the umbilicus. Lhermitte's sign (tingling on neck flexion) can be demonstrated at any stage. Basilar impression can cause headache with coughing, trigeminal neuralgia, loss of function of the extremities, or paresthesias. At its most severe involvement, sleep apnea and death can occur.
Common variable OI with normal sclerae (previously OI type IV) is characterized by mild short stature, DI, adult-onset hearing loss, and normal-to-gray sclerae. This is the most variable form of OI, ranging in severity from moderately severe to so mild that it may be difficult to make the diagnosis.
Stature is variable and may vary markedly within the family. DI is common but may be mild. Sclerae are typically light blue or gray at birth but quickly lighten to near normal. Hearing loss occurs in some and basilar impression can occur.
Other Considerations
Facial features. Infants and children with OI are often described as having a triangular face. The skull is relatively large compared to body size.
Other skeletal problems. Individuals with OI may also have scoliosis, early-onset arthritis, non-inflammatory arthralgia, and myofascial pain.
Skin. Easy bruising is a frequent observation in individuals with OI. This is believed to be caused by microvascular fragility and poor microstructural support of the connective tissues.
Hearing loss. Mixed conductive and sensorineural hearing loss afflicts the majority of adults with OI. Childhood-onset hearing loss affects approximately 7% of affected children between ages five and nine years; progressive postpubertal hearing loss is more typical. The initial conductive hearing loss results from fractures of the bones of the middle ear with contracture and scarring of the incus. With age, sensorineural hearing loss compounds the preexisting conductive element. Fixation of the stapes is not unlike otosclerosis and surgical techniques such as stapedotomy used to treat otosclerosis have shown similar success in treating hearing loss in OI [van der Rijt & Cremers 2003, Kuurila et al 2004, Doi et al 2007]. Bisphosphonate therapy has not been shown to influence hearing loss.
Gastrointestinal. Although complaints of constipation are common in adults with OI who are mobile in wheelchairs, it is not clear if this is a complication of OI itself or of the mode of transport. Bowel obstruction can occur as a result of protrusio acetabuli [Lee et al 1995] but appears to be uncommon.
Cardiovascular. Emerging data support an increased risk for cardiac and vascular disease in OI. Ashournia et al [2015] performed a systematic review of the literature in 2015 documenting a broad array of cardiovascular phenotypes with higher prevalence in individuals with a clinical diagnosis of OI including arterial and aortic dissection. Balasubramanian et al [2019] reported three additional individuals with COL1A1/2-OI and aortic aneurysms. There is still no consensus on cardiovascular surveillance, although some centers have initiated screening echocardiograms every three to five years to monitor for this risk.
Development. Cognition is expected to be normal but gross motor development may be hindered by joint hypermobility and progressive deformity due to recurrent fractures.
Functional limitations. Individuals with OI may experience other functional limitations, although these will be highly dependent on the specific physical manifestations of OI.
Life expectancy. The severely affected neonates with perinatally lethal OI typically do not survive, with a significant proportion of infants dying within the first 48 hours. Aggressive life support can prolong survival but ultimately the most severe forms remain perinatally lethal. Life expectancy for classic non-deforming OI and common variable OI is normal. Progressively deforming OI is highly variable and life expectancy may be shortened by the presence of severe kyphoscoliosis with attendant restrictive pulmonary disease resulting in cardiac insufficiency.
Phenotype Correlations by Gene
Most commonly OI results from pathogenic heterozygous variants in either of the genes encoding the alpha helical chains of type 1 collagen that form the collagen triple helical molecule. Quantitative impacts on type 1 collagen tend to result in a milder phenotype when compared to qualitative changes due to a dominant-negative effect. Loss-of-function variants generally are associated with classic non-deforming OI with blue sclerae (previously OI type I).
In general, a clear genotype-phenotype correlation does not exist. General rules for genotype-phenotype correlations in COL1A1/2-OI have been published [Ben Amor et al 2011], but there are exceptions to these rules (e.g., glycine to serine substitutions may lead to a more severe phenotype in COL1A1 than a similar change in COL1A2). The extent of variation and the clinical presentation is represented in Maioli et al [2019] (see Figure 2).
Genotype-Phenotype Correlations
It is important to keep the exceptions in mind when providing genetic counseling, particularly in the prenatal setting. Genotyping can be helpful in distinguishing classic non-deforming OI from all other types of OI.
Classic non-deforming OI almost always results from a pathogenic variant in one COL1A1 or COL1A2 allele that introduces premature termination codons and decreases the stability of mRNA (nonsense-mediated decay of the message resulting in a quantitative reduction of the collagen fibril). These causative variants may occur by codon changes, by frame shifts, and by splicing that results in use of cryptic splice sites and premature termination. The type I collagen molecule contains two pro α1(I) chains and a single α2(I) chain. If the number of available pro α1(I) chains decreases, the amount of the trimer manufactured is diminished because no more than one pro α2(I) chain can be accommodated per molecule.
Perinatally lethal OI, progressively deforming OI, and common variable OI all result from pathogenic variants that alter the structure of either pro α1(I) or pro α2(I) chains. This causes a dominant-negative effect whereby the abnormal protein is integrated into the triple helix and collagen fibril, which in turn undergoes continual remodeling, thus resulting in significantly compromised structural integrity of the bone matrix (a qualitative impact on the protein product).
The most common pathogenic variants result in substitution of another amino acid for glycine in the triple helical domain of either chain; serine, arginine, cysteine, and tryptophan result from substitutions in the first position of the glycine codon and alanine, valine, glutamic acid, and aspartic acid result from substitutions in the second position of the glycine codon. Glycine is the least bulky amino acid, and other substituting amino acids do not fit well into the collagen triple helix.
- Substitutions in the pro α1(I) chain by arginine, valine, glutamic acid, aspartic acid, and tryptophan are almost always lethal if they occur in the carboxyl-terminal 70% of the triple helix and have a non-lethal but still moderately severe phenotype if they occur in the remainder of the chain.
- For the smaller side-chain residues (serine, alanine, and cysteine), the phenotypes are more variable and appear to reflect some characteristics of the stability profile of the triple helix that are not yet fully recognized.
- Much more variability occurs with pathogenic variants that affect glycine residues in the pro α2(I) chain, even with the large side-chain residues; therefore, it is more difficult to determine the genotype-phenotype relationship.
The other common disease-causing variants affect splice sites. Variants that lead to exon skipping in the pro α1(I) chain beyond exon 14 and in the pro α2(I) chain beyond exon 25 are generally lethal. The phenotypes resulting from pathogenic variants in the upstream region are more variable and may lead to significant joint hypermobility.
A relatively small number of pathogenic variants that alter amino acid sequences in the carboxyl-terminal regions of both chains have been identified. These domains are used for chain association and pathogenic variants have the capacity to destroy this property or lead to abnormalities in chain association. The phenotypic effects of pathogenic variants that affect this domain appear to be milder when they result in exclusion rather than inclusion of the chain.
Somatic mosaicism for dominant pathogenic variants has been recognized in perinatally lethal OI, progressively deforming OI, and common variable OI. The phenotype of the individual with somatic mosaicism can range from no identifiable characteristics of OI to one of the mild forms. The current estimate for the incidence of somatic/gonadal mosaicism is up to 16% of families.
- Individuals with somatic mosaicism for variants that result in non-lethal forms of OI generally have no phenotypic features of OI, even when the variant is present in a majority of somatic cells.
- Somatic mosaicism for variants that result in lethal OI can produce a mild OI phenotype if the variant is present in the majority of somatic cells; otherwise, the mosaicism is generally asymptomatic.
Penetrance
The penetrance in individuals heterozygous for a COL1A1 or COL1A2 pathogenic variant is 100%, although expression may vary considerably, even in the same family.
Nomenclature
Current (and previously used) nomenclature:
- Classic non-deforming OI with blue sclerae (previously, osteogenesis imperfecta type I)
- Perinatally lethal OI (osteogenesis imperfecta type II)
- Progressively deforming OI (osteogenesis type III)
- Common variable OI with normal sclerae (osteogenesis imperfecta type IV)
The classification scheme of "OI congenita" and "OI tarda" was discarded because fractures at birth can be noted in mild OI and infants with severe OI may not have fractures at birth.
In classifications of genetic conditions, OI may be considered a skeletal dysplasia, a connective tissue disorder, a disorder of collagen or extracellular matrix, or a disorder of bone fragility.
Prevalence
Considering all types, OI has a prevalence of approximately 6-7:100,000. COL1A1/2-OI comprises the largest proportion of OI, representing about 90% of all causes of OI.
Differential Diagnosis
Distinguishing COL1A1/2 Osteogenesis Imperfecta (COL1A1/2-OI) from Other Types of OI
The primary differential diagnoses for individuals with features of COL1A1/2-OI are non-collagen-associated forms of OI. There are both dominant and recessive types, which can be phenotypically indistinct from COL1A1/2-OI. In a small subset of individuals, specific causative variants have not yet been identified. Table 5 summarizes the molecular basis of these subtypes of OI, the mode of inheritance, the corresponding clinical OI type, and distinguishing clinical and radiographic features.
Table 5.
Other Types of Osteogenesis Imperfecta in the Differential Diagnosis of COL1A1/2 Osteogenesis Imperfecta
| Functional Group | Gene | MOI | OMIM-Defined Genetic OI Type 1 | Clinical OI Type 2 | Distinguishing from COL1A1/2-OI |
|---|---|---|---|---|---|
| Collagen type I processing | BMP1 | AR | OI type XIII (OMIM 614856) | OI-III | Umbilical hernia; hypertelorism; no DI or HL |
| CRTAP | AR | OI type VII (OMIM 610682) | OI-II, III, or IV | Normal birth length; proptosis; no DI; pulmonary vasculature malformations; rhizomelia | |
| FKBP10 | AR | Bruck syndrome 1 / OI type XI (OMIM 259450 & 610968) | OI-III or IV | Brachycephaly; no easy bruising; no DI or HL; white sclera; inguinal hernia; joint contractures; pterygia | |
| P3H1 (LEPRE1) | AR | OI type VIII (OMIM 610915) | OI-II or III | No DI; white sclerae; proptosis; long phalanges | |
| PLOD2 | AR | Bruck syndrome 2 (OMIM 609220) | OI-III | No DI or HL; white sclerae; inguinal hernia; joint contractures; pterygia | |
| PPIB | AR | OI type IX (OMIM 259440) | OI-II, III, or IV | No DI or HL; white sclerae | |
| SEC24D | AR | Cole-Carpenter syndrome 2 (OMIM 616294) | OI-III | Turricephaly; proptosis; hypertelorism; dysplastic ears; no HL; white sclerae; hydrocephalus; high-pitched voice | |
| SERPINH1 | AR | OI type X (OMIM 613848) | OI-III | Macrocephaly; proptosis; renal calculi | |
| SPARC | AR | OI type XVII (OMIM 616507) | OI-IV | No DI or HL; white sclerae; risk for intracranial hemorrhage | |
| TMEM38B | AR | OI type XIV (OMIM 615066) | OI-III | No DI or HL; white sclerae | |
| Other osteoblast genes | CREB3L1 | AR | OI type XVI (OMIM 616229) | OI-III | Tooth agenesis |
| IFITM5 | AD | OI w/calcification in interosseous membranes, OI type V (OMIM 610967) | Short stature & fractures | Sclerae generally white; DI rare; hypertrophic callus formation; calcification of interosseous membrane between ulna & radius that leads to inability to fully supinate & pronate forearm; no HL | |
| MBTPS2 | XL | OI type XIX (OMIM 301014) | OI-III or IV | No HL; sclerae generally white; rhizomelia; epiphyseal "popcorn" calcification | |
| MESD 3 | AR | OI type XX (OMIM 618644) | OI-III-IV | Facial dysmorphisms incl arched eyebrows & tented shape of lips; long fingers w/5th finger camptodactyly; oligodontia | |
| SERPINF1 | AR | OI type VI (OMIM 613982) | OI-III or IV | No DI or HL | |
| SP7 | AR | OI type XII (OMIM 613849) | OI-III or IV | No DI; white sclerae | |
| TENT5A (FAM46A) | AR | OI type XVIII (OMIM 617952) | OI-III or IV | No DI or HL; umbilical hernia | |
| WNT1 | AR | OI type XV (OMIM 615220) | OI-III or IV | Structural brain malformations; rhizomelia |
AD = autosomal dominant; AR = autosomal recessive; DI = dentinogenesis imperfecta; HL = hearing loss; MOI = mode of inheritance; XL = X-linked
- 1.
See OMIM: Clinical Synopsis Table.
- 2.
Bonafe et al [2015], Robinson & Rauch [2019]
- 3.
Moosa et al [2019]
Distinguishing OI from Other Disorders and Non-Accidental Trauma
The differential diagnosis of OI depends largely on the age at which the individual is assessed [Plotkin 2004]. Clinical features that help to differentiate COL1A1/2-OI from other conditions include characteristic triangular facies, blue sclerae, joint hypermobility, dental abnormalities, and, in adults, hearing loss.
In Utero Assessment
Early prenatal ultrasound examination or radiographic findings may lead to a consideration of hypophosphatasia, thanatophoric dysplasia, campomelic dysplasia, and achondrogenesis as well as perinatally lethal OI. In some cases, either biochemical or molecular testing can be a useful adjunct.
Table 6.
Genes of Interest in the Differential Diagnosis of COL1A1/2 Osteogenesis Imperfecta – In Utero Assessment
| Gene | Differential Diagnosis Disorder 1 | MOI | Prenatal Ultrasound & Radiographic Findings in Differential Diagnosis Disorder | |
|---|---|---|---|---|
| Overlapping w/COL1A1/2-OI | Not observed in COL1A1/2-OI | |||
| ALPL | Perinatal hypophosphatasia | AR | Blue sclerae; abnormal teeth; fractures | Rickets; GI: poor feeding, emesis; craniosynostosis; vertebral clefts; low alkaline phosphatase; high calcium in serum & urine; bone spurs |
| FGFR3 | Thanatophoric dysplasia | AD | In severe OI: fetal hydrops & respiratory distress | Severe micromelia; cloverleaf skull; small iliac bones, narrow sacroiliac notch |
| SLC26A2 | Achondrogenesis type 1B | AR | Thin short ribs in severe OI | Absent mineralization; severe micromelia |
| SOX9 | Campomelic dysplasia | AD | Respiratory distress in severe OI; short limbs & small chest | Curved femurs w/skin dimpling; absent/hypoplastic scapula; macrocephaly; large anterior fontanelle; male sex reversal (46XY, female); undermineralized thoracic vertebral pedicles; congenital heart defect |
AD = autosomal dominant; AR = autosomal recessive; MOI = mode of inheritance
- 1.
See OMIM: Clinical Synopsis Table.
Infancy and Childhood Assessment
Non-accidental trauma (NAT; child abuse). COL1A1/2-OI needs to be distinguished from child physical abuse / non-accidental trauma. The prevalence of physical abuse is much greater than the prevalence of COL1A1/2-OI, and on rare occasion, it can occur in a child with COL1A1/2-OI. Patient history, family history, physical examination, radiographic imaging, and the clinical course all contribute to distinguishing COL1A1/2-OI from child abuse. The overlap in clinical features includes multiple or recurrent fractures, fractures that do not match the history of trauma, and the finding of fractures of varying ages and at different stages of healing [Carty 1988, Ablin et al 1990, Steiner et al 1996, Ablin & Sane 1997, Marlowe et al 2002].
The continued occurrence of fractures in a child who has been removed from a possibly abusive situation lends support to the possibility of COL1A1/2-OI. Metaphyseal and rib fractures, thought to be virtually pathognomonic for child abuse, can rarely occur in COL1A1/2-OI. The presence or absence of blue sclerae is unreliable in distinguishing COL1A1/2-OI from child abuse because blue sclerae are often found in unaffected normal infants until about age 18 months; children with OI type IV may not have blue sclerae.
Family history is often unrevealing; families suspected of possible child abuse often provide an unverified family history of frequent fractures; conversely, the family history of individuals with COL1A1/2-OI often does not reveal any other affected individuals because of a de novo pathogenic variant in the proband or the presence of a mild phenotype in relatives.
Laboratory testing (typically molecular genetic testing of COL1A1 and COL1A2) usually is not needed to differentiate COL1A1/2-OI from NAT, and in some cases, the time required to perform such testing can delay proper disposition of child abuse cases [Steiner et al 1996]. Marlowe and colleagues suggest: "Given the inability to identify all children with OI by clinical examination in situations of suspected non-accidental injury, laboratory testing for OI (and other genetic predispositions for fractures) is a valuable adjunct in discerning the basis for fractures and may identify a small group of children with previously undiagnosed OI" [Marlowe et al 2002]. Laboratory testing in such cases is no substitute for proper clinical evaluation that includes history, family history, physical examination, and radiographic evaluation.
Other genetic disorders. See Table 7.
Table 7.
Other Genetic Disorders of Interest in the Differential Diagnosis of COL1A1/2 Osteogenesis Imperfecta
| Gene | Differential Diagnosis Disorder 1 | MOI | Clinical Features of the Differential Diagnosis Disorder | |
|---|---|---|---|---|
| Overlapping w/COL1A1/2-OI | Not observed in COL1A1/2-OI | |||
| ANO5 | Gnathodiaphyseal dysplasia (OMIM 166260) | AD | Osteopenia & bone fragility | Enlarged jaw; osteomyelitis |
| DSPP | Dentinogenesis imperfecta (OMIM 125490) | AD | DI | Primary teeth more impacted than secondary teeth |
| GORAB | Gerodermia osteodysplastica (OMIM 231070) | AR | Osteopenia & bone fragility | Microcephaly; premature aged facial appearance; camptodactyly; intellectual disability |
| LRP5 | Osteoporosis pseudoglioma syndrome (OMIM 259770) | AR | Osteopenia & bone fragility | Microcephaly; pseudoglioma; cataracts; blindness; congenital heart defect |
| NOTCH2 | Hadju-Cheney syndrome (OMIM 102500) | AD | Osteopenia & bone fragility | Coarse facial appearance; early tooth loss; congenital heart defect; male hypospadias/cryptorchidism; renal cysts |
| P4HB | Cole-Carpenter syndrome 1 (OMIM 112240) | AD | Enamel hypoplasia; osteopenia & fractures | Midface hypoplasia; frontal bossing; proptosis; pseudoclubbing; acroosteolysis |
AD = autosomal dominant; MOI = mode of inheritance
- 1.
See OMIM: Clinical Synopsis Table.
Idiopathic juvenile osteoporosis (OMIM 259750) typically presents in pre-adolescents with fractures and osteoporosis. The fracture susceptibility and osteoporosis usually resolve spontaneously with puberty.
Management
Evaluations Following Initial Diagnosis
To establish the extent of disease and needs of an individual diagnosed with COL1A1/2 osteogenesis imperfecta (COL1A1/2-OI), the evaluations summarized in Table 8 (if not performed as part of the evaluation that led to the diagnosis) are recommended.
Table 8.
Recommended Evaluations Following Initial Diagnosis in Individuals with COL1A1/2 Osteogenesis Imperfecta
| System/Concern | Evaluation | Comment |
|---|---|---|
| Musculoskeletal | Physical examination | To assess deformities & presence of joint laxity |
| As indicated by clinical presentation | |
| Neurologic | CT &/or MRI examination w/views across base of skull to evaluate for basilar impression | If concerning signs or symptoms are present 1 |
| Cervical spine flexion & extension radiographs | In children able to cooperate w/examination or before participating in sporting activities in more mildly affected individuals | |
| Dental | Dental examination |
|
| Audiologic | Formal hearing assessment | In all individuals at diagnosis |
| Other | Consultation w/clinical geneticist &/or genetic counselor |
DI = dentinogenesis imperfecta; OT = occupational therapist; PT = physical therapist
- 1.
There is no universal agreement on when screening for basilar impression should be performed. A positive "Lhermitte's sign" (tingling in fingers with neck flexion) should prompt neurosurgical referral. Surgery is typically undertaken before persistent/permanent neurologic features are present.
Treatment of Manifestations
Management focuses on supportive therapy to minimize fractures and maximize function, minimize disability, foster independence, and maintain overall health [Marini & Gerber 1997]. Ideally, COL1A1/2-OI is managed by a multidisciplinary team including specialists in the medical management of COL1A1/2-OI, clinical genetics, orthopedics, rehabilitation medicine, pediatric dentistry, otology/otolaryngology, and mental health.
Supportive therapy is individualized depending on the severity, degree of impairment, and age of the affected individual. Considerable support from medical personnel is generally required by parents caring for infants with perinatally lethal COL1A1/2-OI.
Physical medicine treatment
- Parents and other caregivers should be instructed in safe handling techniques. These are mostly commonsense practices in order to relieve stress on a single point. For example: lift an affected infant by bracing the torso, neck, and lower body; avoid any situation where increased pressure is placed on a single point on any long bone; when assisting an affected child in standing up, do not pull excessively on an extended arm but bend down and brace a greater surface area (e.g., placing a hand behind the back and pulling gently from the front – using the arm – while applying pressure from the rear); avoid sudden acceleration/deceleration movements; and avoid throwing a child in the air. To minimize point pressure, avoid lifting an infant by the ankle when diapering. Older children should not ride on amusement park rides. Caregivers should avoid re-creating the circumstances of a fracture, as it is likely to happen again.
- The use of bracing to try to stabilize progressively deforming limbs depends in part on the subtype of COL1A1/2-OI. Progressively deforming OI has proven to be progressive despite external or internal bracing. The use of internal rods or braces to support and stabilize deforming limbs is more successful in the milder subtypes of COL1A1/2-OI and is guided by the expertise of the managing orthopedist.
- Orthotics to support ankle instability are used in toddlers with delayed walking secondary to joint hypermobility and in other affected individuals who suffer recurrent subluxations of their ankle joints.
- Physical activity serves a number of purposes. It provides gravitational stressors required for bone growth and remodeling. The muscles' supporting joints are strengthened by activity, and as an overall benefit, improved joint stability aids in overall well-being as pain levels are reduced and mobility is increased. Physical activity can be self directed or coordinated through the services of a physical therapist. Each affected individual's needs are unique and thus both physical and occupational therapy should be initiated for increased stability of bone, improved mobility, prevention of contractures, prevention of head and spinal deformity, and improved aerobic fitness and muscle strengthening.
- Mobility devices, such as scooters and chairs for children and modified automobiles for adults, should be considered.
- Some individuals with COL1A1/2-OI experience chronic daily pain associated with both fractures and nonspecific myofascial pain associated with the generalized connective tissue disorder. Pain management plays an important role in the management of COL1A1/2-OI. Some affected individuals do well with minimal analgesics, but many benefit from a multidisciplinary pain management service. Analgesics can be used to control pain from fractures.
Orthopedic treatment. Fractures are treated as they would be in unaffected children and adults with attention to the following:
- The period of immobility in children with COL1A1/2-OI should be shortened as much as is practical.
- Casts should be small and lightweight.
- Physical therapy should begin as soon as the cast is removed to promote mobility and enhance muscle strength and bone mass.
- At this time, intramedullary rodding remains a mainstay of orthopedic care to provide anatomic positioning of limbs to permit more normal function.
Progressive spinal deformities are particularly difficult to treat because of the poor quality of bone in severely affected children. Progressive scoliosis in severe COL1A1/2-OI may not respond well to conservative management and response to surgical intervention may be limited.
Pharmacologic treatment. Bisphosphonates, analogs of pyrophosphate that decrease bone resorption, are being evaluated in both uncontrolled and controlled trials to assess the extent to which they can increase bone mass and bone strength and improve function in children with COL1A1/2-OI. These studies are still ongoing. Bisphosphonates have been used most extensively in severely affected children; they may be useful in adults as well [Adami et al 2003].
The role of treatment with bisphosphonates in changing the natural history of COL1A1/2-OI is incompletely understood. The Cochrane Collaboration is an international network that assembles reviews on various management strategies based on randomized controlled clinical trials within its database in order to improve the practice of evidence-based medicine. As of the Cochrane Collaboration's most recent update of the OI review, bisphosphonate therapy did not appear to reduce fracture incidence but it did affect bone density and adult height [Dwan et al 2014] (full text).
In a more recent publication Bains et al [2019] collated data from the Osteogenesis Imperfecta Foundation's linked clinical research centers on 466 patients with all forms of OI who had been treated with bisphosphonates. The review of the data primarily focused on classic non-deforming OI with blue sclerae (type I). Primary findings indicated increased lumbar vertebral body density, and statistical regression analysis indicated reduced probability of fracture and scoliosis in individuals treated with bisphosphonates compared with those untreated.
Pamidronate use is invasive and typically requires intravenous infusions every three months, four hours a day, for three days. Pamidronate has been used to treat newborn infants with severe OI; complications include transient asymptomatic hypocalcemia [Plotkin et al 2000] and symptomatic hypocalcemia [Chien et al 2002]. The long-term consequences of lowering bone turnover in children with COL1A1/2-OI are unknown but may include delayed bone union after fracture or osteotomy.
A randomized controlled clinical trial using the oral bisphosphonate alendronate found that treatment with oral alendronate for two years in children with OI significantly decreased bone turnover and increased spine areal BMD (bone mineral content measured by DEXA divided by bone area in square centimeters) but was not associated with improved fracture outcomes [Ward et al 2011]. In a second study with a different oral bisphosphonate, Bishop and colleagues found that oral risedronate increased areal BMD and reduced first and recurrent clinical fractures in children with OI [Bishop et al 2010]. Zoledronic acid, a bisphosphonate with a longer half-life, greater potency, and more convenient dosing, has been studied in children with OI. Lv et al [2018] compared the efficacy of an annual infusion of zoledronic acid to weekly oral alendronate and concluded that these treatment approaches had similar increases in vertebral BMD and were well tolerated.
Basilar impression. It is important to screen for this finding so that timely surgical intervention can be planned. A positive "Lhermitte's sign" (tingling in fingers with neck flexion) should prompt neurosurgical referral. Surgery is typically undertaken before persistent/permanent neurologic features are present. If surgery is undertaken, it should be done in a center experienced in the procedures used.
Dental treatment. The goals are the maintenance of both primary and permanent dentition, functional bite or occlusion, optimal gingival health, and overall appearance. Pediatric dentists are the most knowledgeable about dentinogenesis imperfecta (DI) in children. Some consensus exists that early dental restorative coverage of the primary molars and (if possible) aesthetic coverage of the upper anterior teeth is optimal. Plastic polymers are sometimes used to coat teeth. As anxiety can be an issue with children, pre-medication for anxiolysis (e.g., nitrous oxide analgesia or midazolam) can be used for treatment in a clinic setting.
If warranted, orthodontic treatment can be initiated, but care must be taken in the use of orthodontic appliances because of the brittleness of the teeth.
Dental restorations in adults may best be done by a general dentist knowledgeable about OI or a specialist in prosthetic dentistry.
Hearing loss. Surgical repair of the middle-ear bones and creation of a prosthetic incus can improve unaided hearing.
Later hearing loss appears to have a significant sensorineural component that does not respond to middle ear surgery. Cochlear implantation has been used in a small number of individuals; outcome data are limited.
Mental health support through psychiatry/psychology and appropriate social worker intervention can improve quality of life.
Management of lethal OI. It is appropriate to offer parents the option of allowing the infant to expire without attempting heroic interventions such as assisted ventilation.
Other therapies. Early trials of anabolic steroids, sodium fluoride, testosterone, vitamins C and D, flavinoids, and calcitonin showed minimal or no improvement in bone formation, or too small a sample size was utilized for meaningful conclusions [reviewed in Byers & Steiner 1992].
Prevention of Secondary Complications
Special attention should be paid to anesthesia concerns including proper positioning on the operating room table, for which egg crate foam is recommended to avoid fractures.
Surveillance
Table 9.
Recommended Surveillance for Individuals with COL1A1/2 Osteogenesis Imperfecta
| System/Concern | Evaluation | Frequency |
|---|---|---|
| Musculoskeletal | Orthopedic evaluation | Every 3 mos until age 1 yr, every 6 mos from 1 to 3 yrs, then annually or w/any new fractures |
| Physical & rehabilitation medicine | Annually or more frequently if necessary for more severe clinical forms | |
| Physical therapy | In infancy for individuals w/motor delays & as needed to improve mobility & function | |
| Neurologic | CT &/or MRI examination w/views across base of skull to evaluate for basilar impression | If concerning signs or symptoms are present 1 |
| Cervical spine flexion & extension radiographs | In children able to cooperate w/examination or before participating in sporting activities in more mildly affected individuals | |
| Dental | Dental examinations | 2x/yr for those w/DI or at risk for DI |
| Audiologic | Hearing evaluation | Every 3-5 yrs from age 5 yrs until HL is identified, then as indicated based on nature & degree of HL & associated interventions |
DI = dentinogenesis imperfecta; HL = hearing loss
- 1.
There is no universal agreement on when screening for basilar impression should be performed.
Agents/Circumstances to Avoid
Contact sports should be avoided.
Evaluation of Relatives at Risk
It is appropriate to clarify the genetic status of apparently asymptomatic older and younger at-risk relatives of an affected individual in order to identify as early as possible those who would benefit from cervical spine examination and dental and hearing evaluations.
See Genetic Counseling for issues related to testing of at-risk relatives for genetic counseling purposes.
Pregnancy Management
Fertility is normal in OI. Pregnancy in women with OI, especially those with progressively deforming OI, can be complicated because of a small pelvis, which may necessitate delivery by cesarean section. For most women who have mild non-deforming OI, pregnancy is uncomplicated. Joint laxity may increase, as it does with unaffected women, and reduce mobility in small, moderately affected women. Bleeding is not more common than usual and complications of vaginal tearing during delivery are not common. Women with OI who are very small require pre-term cesarean section because of respiratory compromise. It is uncertain whether postpartum pelvic relaxation is more common. The mode of delivery of infants with OI has been examined to determine if the frequency of complications is higher with vaginal or cesarean section delivery. No difference in the frequency of complications was found. A higher-than-expected frequency of non-vertex presentations has been noted [Cubert et al 2001]. The role of pregnancy in later fractures, loss of bone mineralization, progression of hearing loss, or any other physical consideration has not been examined in detail.
Women with OI who have significant skeletal deformity and short stature should be followed during pregnancy at a high-risk prenatal care center.
Cesarean section and vaginal delivery of an infant with OI have about the same rate of complications for each type of OI. Delivery of an infant with OI by cesarean section is more frequent than in the general population because a non-vertex presentation cannot be corrected by external manipulation.
Therapies Under Investigation
RANK ligand antibodies, which inhibit osteoclast maturation, have been studied as a therapeutic option in children and results have shown an increase in vertebral body BMD, normalization of vertebral shape, and a reduction in vertebral compression fractures while on therapy. Ongoing studies are determining long-term efficacy and tolerance [Hoyer-Kuhn et al 2014, Hoyer-Kuhn et al 2016, Boyce 2017].
Human growth hormone has been evaluated as an adjunctive therapy in conjunction with bisphosphonates in a randomized controlled study. In this study, growth hormone therapy was reported to correlate with improved linear growth and increased BMD [Antoniazzi et al 2010]. An additional study presented similar results in 26 children with moderate to severe OI when growth hormone was used in isolation [Marini et al 2003].
Teriparatide, a PTH analog, has been used to treat osteoporosis and is being explored as an adjunctive therapy in OI [Orwoll et al 2014]. The risk for osteosarcoma in those treated with teriparatide has limited its widespread use in children with more severe forms of OI. An ongoing registry monitoring the risk in adults treated with teriparatide has not documented an instance of osteosarcoma in the eight years since it was established but longer observation is needed to identify the actual risk of osteosarcoma [Gilsenan et al 2018].
Bone marrow transplantation (BMT) to introduce normal mesenchymal stem cells that have the capacity to differentiate into normal osteoblasts as well as transplanted mesenchymal stromal cells, which produce factors that stimulate endogenous bone growth in individuals with OI, has been evaluated in a pilot clinical trial. Preliminary data suggested a positive impact on growth [Otsuru et al 2012, www.clinicaltrials.gov].
Search ClinicalTrials.gov in the US and EU Clinical Trials Register in Europe for information on clinical studies for a wide range of diseases and conditions.