Multiple Enchondromatosis, Maffucci Type
Description
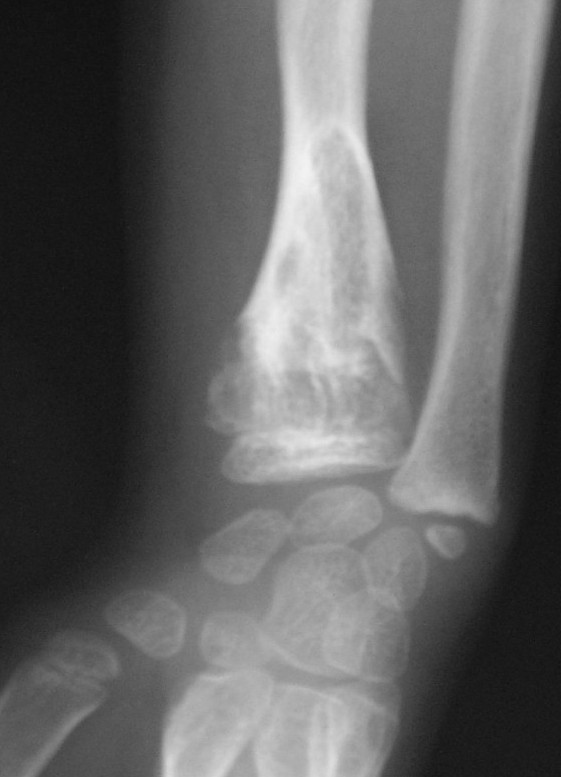
Enchondromas are common benign cartilage tumors of bone. They can occur as solitary lesions or as multiple lesions in enchondromatosis. When hemangiomata are associated, the condition is known as Maffucci syndrome. Clinical problems caused by enchondromas include skeletal deformity and the potential for malignant change to osteosarcoma (Schwartz et al., 1987).
Classification of the Enchondromatoses
In their classification of the enchondromatoses, Spranger et al. (1978) called Ollier disease (166000) and Maffucci syndrome types I and II enchondromatosis, respectively; metachondromatosis (156250), type III; and spondyloenchondrodysplasia (607944), type IV; enchondromatosis with irregular vertebral lesions, type V; and generalized enchondromatosis, type VI. Halal and Azouz (1991) added 3 tentative categories to the 6 in the classification of Spranger et al. (1978).
Pansuriya et al. (2010) suggested a new classification of enchondromatosis (multiple enchondromas).
Clinical FeaturesKaplan et al. (1993) reported 2 men, aged 42 and 44 years, with Maffucci syndrome. The latter had had multiple enchondromas and subcutaneous hemangiomas since birth on both the arms and the legs. A painful mass that had been growing in the region of the right elbow in recent months was found to be a chondrosarcoma. The patient underwent right shoulder disarticulation. Kaplan et al. (1993) reviewed 63 other cases, concluding that the syndrome is not hereditary, occurs in all races, and occurs in both sexes equally. They suggested that chondrosarcoma occurs in 30% of patients with Maffucci syndrome. As in the case of multiple exostoses and neurofibromatosis type I, the frequency of malignancy may be exaggerated through bias; the patients in whom malignancy develops are, of course, more likely to come to medical attention.
Lee et al. (1999) reported a Korean girl with histologically confirmed, multiple hemangiomas on the left palm, sole, and face, and multiple endochondromas in the skeletal system. Because of mild anemia and occult blood in the stool, upper gastrointestinal endoscopy and sigmoidoscopy were carried out, which revealed hemangiomas in the hypopharynx and the ascending colon. Hemangiomas in such locations are rare in Maffucci syndrome.
InheritanceMost cases of Maffucci syndrome have been sporadic (Halal and Azouz, 1991).
Population GeneticsSun et al. (1985) reported that 9 patients with Maffucci syndrome seen at the Mayo Clinic developed chondrosarcoma. From a review of the English literature since 1973, they concluded that the incidence of chondrosarcoma in this disorder is 17.8%. This conclusion is suspect. The difficulties of stating the frequency of malignancy in von Recklinghausen neurofibromatosis and multiple exostoses from hospital records or reports is well known.
Molecular GeneticsIn enchondromas and chondrosarcomas from 31 enchondromatosis patients (Ollier disease or Maffucci syndrome, lacking platyspondyly) from 3 different European countries, Rozeman et al. (2004) failed to find evidence of the R150C mutation in the PTHR1 gene (168468) described by Hopyan et al. (2002) in a patient with Ollier disease, nor did they find any other mutation in the PTHR1 gene. Immunohistochemistry revealed normal expression of the PTHR1 protein. Rozeman et al. (2004) concluded that enchondromatosis is not caused by the R150C PTHR1 mutation, although they stated that the discrepancy in findings may have been caused by the R150C mutation being a founder mutation in the Canadian population, or may reflect that the patients of Hopyan et al. (2002) belonged to a different, rare subclass of enchondromatosis instead of having Ollier disease.
Couvineau et al. (2008) analyzed the coding sequence of PTHR1, IHH (600726), PTHRP (168470), and GNAS1 (139320) in leukocyte and/or tumor DNA from 61 and 23 patients affected with Ollier disease or Maffucci syndrome, respectively. No deleterious mutations were identified among the patients with Maffucci syndrome.
Pansuriya et al. (2011) reported somatic heterozygous mutations in IDH1 (147700; 395G-T; R132H) or IDH2 (147650; 516G-C; R172S) in 87% of enchondromas and in 70% of spindle cell hemangiomas. In total, 35 of 43 (81%) individuals with Ollier disease and 10 of 13 (77%) with Maffucci syndrome carried IDH1 (98%) or IDH2 (2%) mutations in their tumors. Eight tumor samples had subthreshold peaks at the position in IDH1 expected to encode mutations resulting in R132C or R132H substitutions and mutations were confirmed in 7 of these tumors by the hydrolysis probe assay. Pansuriya et al. (2011) showed that IDH1 mutations in cartilage tumors were associated with hypermethylation and downregulated expression of several genes. Mutations were absent in DNA isolated from the blood, muscle, or saliva of the subjects. Fourteen of 16 subjects had identical mutations in separate lesions. Of 68 tumors from subjects with Ollier disease, 17 (25%) showed mutant protein expression, whereas 51 (75%) were negative. Within tumors that were positive for IDH1 R132H staining, Pansuriya et al. (2011) observed a mixture of cells that did and did not express the mutant protein, a pattern that the authors referred to as intraneoplastic mosaicism. Within these tumors, the percentage of tumor cells staining positive for IDH1 R132H ranged from 50 to 95%. Pansuriya et al. (2011) also reported mutations in IDH1 or IDH2 in 40 of 101 (40%) solitary central tumors, 7 of 13 (54%) dedifferentiated chondrosarcomas, and 3 of 3 (100%) periosteal chondrosarcomas.
Amary et al. (2011) analyzed 74 tumors from 40 individuals (32 with Ollier disease, 8 with Maffucci syndrome) for mutations in IDH1 (altering arg132) and IDH2 (altering arg140 and arg172). A large proportion (90.5%) of the tumors harbored one of these mutations: 62 of 68 cartilaginous tumors had an IDH1 mutation, and 1 tumor had an IDH2 mutation. Samples from multiple tumors (range, 2-6; mean, 2.8 per subject) were available from 19 of the 40 individuals. Of these 19, 15 were found to have the same mutation in each of their tumor samples that were examined. Tumors from 3 individuals (2 tumors each) had wildtype IDH1 and IDH2 sequences (subjects 2, 10, and 25). One tumor in subject 21 harbored a mutation causing an R132S substitution, whereas the second tumor had wildtype sequences. Sequencing all coding regions of IDH1, IDH2, and PTH1R for the 3 tumors with wildtype sequences for IDH1 and IDH2 from which frozen tissue was available for such analysis did not reveal mutations. The rare IDH1, IDH2, and PTH1R mutations previously reported in these genes were not detected in the remaining 4 paraffin-embedded tumors with wildtype sequences. Additional sequencing of 12 of the 62 tumors with IDH1 mutations did not reveal common PTH1R mutations.
Amary et al. (2011) detected the same R132C mutation in both nonlesional tissue and tumors from 2 of 12 subjects with Ollier disease (subject 1, bone marrow; subject 6, blood), using a custom-made Taqman assay and MassARRAY but not by capillary sequencing. Amary et al. (2011) also found a strong correlation between the presence of mutations and high levels of 2HG and between the absence of mutations and low 2HG levels in a series of central cartilaginous tumors and 1 hemangioma derived from subjects with Ollier disease or Maffucci syndrome and from those with solitary neoplasms (p less than 0.0001). However, 2 tumors with wildtype sequences from a subject with Maffucci syndrome had high levels of 2HG, and the third tumor with wildtype sequences, from a subject with multiple tumors (subject 2), had low levels of 2HG. Amary et al. (2011) suggested a model in which IDH1 mutations are early post-zygotic events in individuals with these syndromes, implying that the mutations are required for tumorigenesis.