Waardenburg Syndrome Type I
Summary
Clinical characteristics.
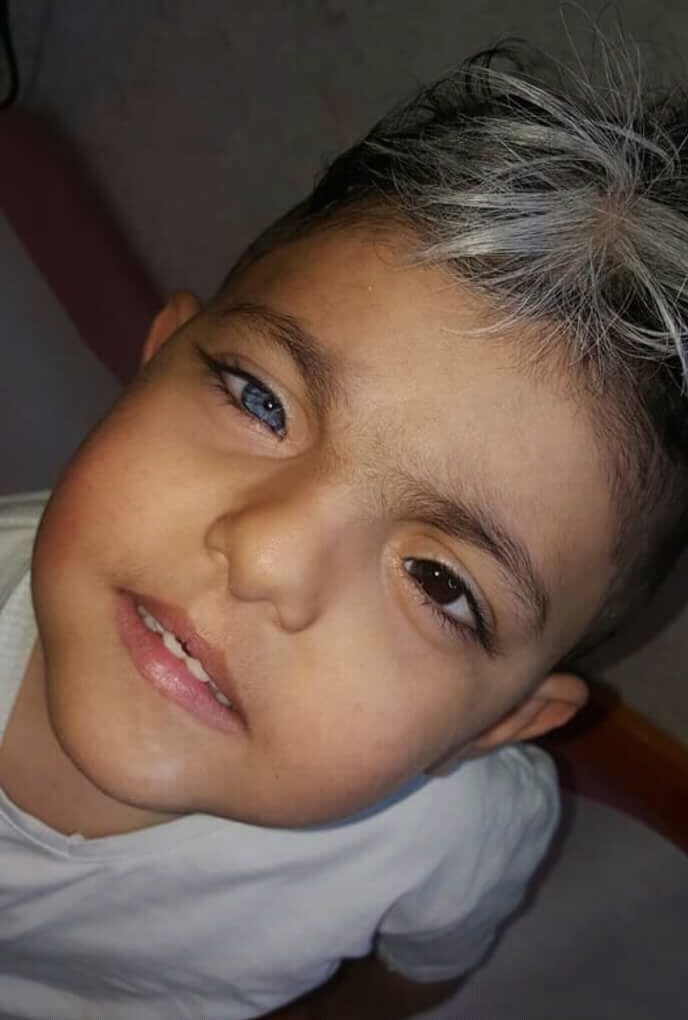
Waardenburg syndrome type I (WS1) is an auditory-pigmentary disorder comprising congenital sensorineural hearing loss and pigmentary disturbances of the iris, hair, and skin, along with dystopia canthorum (lateral displacement of the inner canthi). The hearing loss in WS1, observed in approximately 60% of affected individuals, is congenital, typically non-progressive, either unilateral or bilateral, and sensorineural. Most commonly, hearing loss in WS1 is bilateral and profound (>100 dB). The majority of individuals with WS1 have either a white forelock or early graying of the scalp hair before age 30 years. The classic white forelock observed in approximately 45% of individuals is the most common hair pigmentation anomaly seen in WS1. Affected individuals may have complete heterochromia iridium, partial/segmental heterochromia, or hypoplastic or brilliant blue irides. Congenital leukoderma is frequently seen on the face, trunk, or limbs.
Diagnosis/testing.
The diagnosis of WS1 is established in most individuals by physical examination for clinical criteria including: sensorineural hearing loss, pigmentary changes in the hair and eyes, dystopia canthorum identified by calculation of the W index, and specific facial features. Identification of a heterozygous PAX3 pathogenic variant by molecular genetic testing establishes the diagnosis if clinical features are inconclusive.
Management.
Treatment of manifestations: Management of the hearing loss depends on its severity; cochlear implantation has been successfully used in individuals with WS1.
Evaluation of relatives at risk: If the family-specific PAX3 pathogenic variant is known, molecular genetic testing of relatives at risk allows for early screening of those at risk for hearing loss.
Pregnancy management: Folic acid supplementation in pregnancy is recommended for women at increased risk of having a child with WS1 because of possibly increased risk for neural tube defects in association with WS1.
Genetic counseling.
Waardenburg syndrome type I (WS1) is inherited in an autosomal dominant manner. The majority of probands have an affected parent. A minority of probands do not have an affected parent and are presumed to have WS1 as a result of a de novo pathogenic variant. Offspring of an individual with WS1 have a 50% chance of inheriting the pathogenic variant. If the pathogenic variant has been identified in an affected family member, prenatal testing for pregnancies at increased risk may be available from a clinical laboratory that offers either testing for this disease/gene or custom prenatal testing. Although this testing can determine whether the fetus has inherited the PAX3 pathogenic variant, it cannot determine the clinical manifestations or their severity.
Diagnosis
Suggestive Findings
Waardenburg syndrome type I (WS1) should be suspected in individuals with several of the following major and minor criteria.
Major criteria
- Congenital sensorineural hearing loss
- White forelock, hair hypopigmentation
- Pigmentation abnormality of the iris:
- Complete heterochromia iridum (irides of different color)
- Partial/segmental heterochromia (two different colors in same iris, typically brown and blue)
- Hypoplastic blue irides or brilliant blue irides
- Dystopia canthorum, W index >1.95 (see Note – W index)
- Affected first-degree relative
Minor criteria
- Skin hypopigmentation (congenital leukoderma)
- Synophrys and/or medial eyebrow flare
- Broad/high nasal root, low-hanging columella
- Underdeveloped alae nasi
- Premature gray hair (age <30 years)
Note – W index: The measurements necessary to calculate the W index (in mm) are as follows: inner canthal distance (a), interpupillary distance (b), and outer canthal distance (c).
Calculate X = (2a – (0.2119c + 3.909))/c
Calculate Y = (2a – (0.2479b + 3.909))/b
Calculate W = X + Y + a/b
Click here to download a tool (xlsx) for calculating the W index.
Establishing the Diagnosis
The diagnosis of WS1 is established in a proband with two major criteria or one major plus two minor criteria (see Suggestive Findings) as proposed by the Waardenburg Consortium [Farrer et al 1992].
Identification of a heterozygous pathogenic variant in PAX3 by molecular genetic testing (see Table 1) confirms the diagnosis if clinical features are inconclusive.
Molecular genetic testing approaches can include single-gene testing, a multigene panel, and more comprehensive genomic testing:
- Single-gene testing. Sequence analysis of PAX3 is performed first and followed by gene-targeted deletion/duplication analysis if no pathogenic variant is found.
- A multigene panel that includes PAX3 and other genes of interest (see Differential Diagnosis) may also be considered. Note: (1) The genes included in the panel and the diagnostic sensitivity of the testing used for each gene vary by laboratory and are likely to change over time. (2) Some multigene panels may include genes not associated with the condition discussed in this GeneReview; thus, clinicians need to determine which multigene panel is most likely to identify the genetic cause of the condition at the most reasonable cost while limiting identification of variants of uncertain significance and pathogenic variants in genes that do not explain the underlying phenotype. (3) In some laboratories, panel options may include a custom laboratory-designed panel and/or custom phenotype-focused exome analysis that includes genes specified by the clinician. (4) Methods used in a panel may include sequence analysis, deletion/duplication analysis, and/or other non-sequencing-based tests.For an introduction to multigene panels click here. More detailed information for clinicians ordering genetic tests can be found here.
- More comprehensive genomic testing (when available) including exome sequencing and genome sequencing may be considered if single-gene testing (and/or use of a multigene panel that includes PAX3) fails to confirm a diagnosis in an individual with features of WS1. Such testing may provide or suggest a diagnosis not previously considered (e.g., mutation of a different gene or genes that results in a similar clinical presentation).For an introduction to comprehensive genomic testing click here. More detailed information for clinicians ordering genomic testing can be found here.
Table 1.
Molecular Genetic Testing Used in Waardenburg Syndrome Type I
| Gene 1 | Method | Proportion of Probands with a Pathogenic Variant 2 Detectable by Method |
|---|---|---|
| PAX3 | Sequence analysis 3 | >90% 4 |
| Gene-targeted deletion/duplication analysis 5 | ~6% 6 |
- 1.
See Table A. Genes and Databases for chromosome locus and protein.
- 2.
See Molecular Genetics for information on allelic variants detected in this gene.
- 3.
Sequence analysis detects variants that are benign, likely benign, of uncertain significance, likely pathogenic, or pathogenic. Pathogenic variants may include small intragenic deletions/insertions and missense, nonsense, and splice site variants; typically, exon or whole-gene deletions/duplications are not detected. For issues to consider in interpretation of sequence analysis results, click here.
- 4.
Pingault et al [2010], Milunsky [2011, unpublished data], Wildhardt et al [2013]
- 5.
Gene-targeted deletion/duplication analysis detects intragenic deletions or duplications. Methods used may include quantitative PCR, long-range PCR, multiplex ligation-dependent probe amplification (MLPA), and a gene-targeted microarray designed to detect single-exon deletions or duplications.
- 6.
Milunsky et al [2007]. Note: No duplications have been reported.
Clinical Characteristics
Clinical Description
The phenotype of Waardenburg syndrome type I (WS1) is variable even within a family. Liu et al [1995] summarized the penetrance (percentage) of clinical features of WS1 (see Table 2) in 60 individuals with WS1 and 210 affected individuals reported elsewhere in the literature. Newton [2002] reviewed the clinical features of the Waardenburg syndromes and Tamayo et al [2008] discussed their screening program for Waardenburg syndrome in Colombia, detailing the percentage of each clinical manifestation; percentages similar to those found in the Liu et al [1995] study were documented. However, ascertainment bias was evident, as all 95 affected individuals had hearing loss and were among the institutionalized deaf population in Colombia.
Table 2.
Penetrance of Individuals with Waardenburg Syndrome Type I with Characteristic Clinical Findings
| Clinical Finding | % of Affected Individuals |
|---|---|
| Sensorineural hearing loss | 47%-58% |
| Heterochromic irides | 15%-31% |
| Hypoplastic blue irides | 15%-18% |
| White forelock | 43%-48% |
| Early graying | 23%-38% |
| Leukoderma | 22%-36% |
| High nasal root | 52%-100% |
| Medial eyebrow flare | 63%-73% |
Based on Liu et al [1995], Pardono et al [2003], Tamayo et al [2008]
Hearing loss. The hearing loss in WS1 is congenital, typically non-progressive, either unilateral or bilateral, and of the sensorineural type. The most common type in WS1 is profound bilateral hearing loss (>100 dB). The laterality of the hearing loss shows both inter- and intrafamilial variation.
Various temporal bone abnormalities have been identified in persons with WS1 and hearing loss [Madden et al 2003]. The temporal bone abnormalities include enlargement of the vestibular aqueduct and upper vestibule, narrowing of the internal auditory canal porus, and hypoplasia of the modiolus.
Hair color. The classic white forelock is the most common hair pigmentation anomaly seen in WS1; it may be present at birth, or appear later, typically in the teen years. The white forelock may become normally pigmented over time. The white forelock is typically in the midline but the patch of white hair may also be elsewhere. In evaluating an individual with suspected WS1 without a white forelock, the individual should be asked whether the hair has been dyed. Red and black forelocks have also been described. The majority of individuals with WS1 have either a white forelock or early graying of scalp hair before age 30 years [Farrer et al 1992].
The hypopigmentation can also involve the eyebrows and eyelashes.
Ocular findings. Individuals with WS1 may have a variety of ocular pigmentary manifestations. The most commonly observed are complete or segmental heterochromia or hypoplastic or brilliant blue irides. Iris and choroidal hypopigmentation (sector pattern more than diffuse pattern) has been described [Shields et al 2013]. Visual acuity does not differ from the general population.
Skin pigmentation. Congenital leukoderma (white skin patches) is frequently seen in WS1 on the face, trunk, or limbs. These areas of hypopigmentation frequently have hyperpigmented borders and may be associated with an adjacent white forelock.
Occasional findings identified in multiple families (although too few to determine the percentage occurrence in this disorder):
- Cleft lip and palate
- Spina bifida, a finding that is not surprising given that WS1 is considered a neurocristopathy with PAX3 being expressed in the neural crest. Kujat et al [2007] described the prenatal diagnosis of spina bifida in a family with WS1. Lemay et al [2015] reported a de novo pathogenic nonsense PAX3 variant in an individual with myelomeningocele and WS1.
- Vestibular symptoms including vertigo, dizziness, and balance difficulties, even without hearing loss [Black et al 2001]
Otopathology. The otopathology of an individual with WS1 and a PAX3 pathogenic variant has been described [Merchant et al 2001]. The findings are consistent with defective melanocyte migration or function resulting in defective development of the stria vascularis leading to sensorineural hearing loss.
Genotype-Phenotype Correlations
PAX3. Genotype/phenotype correlations in PAX3 are not well established except for the p.Asn47His pathogenic variant, which causes WS3 [Hoth et al 1993], and the p.Asn47Lys pathogenic variant described in craniofacial-deafness-hand syndrome [Asher et al 1996]. DeStefano et al [1998] found that the presence of pigmentary disturbances in individuals with WS1 correlated more with PAX3 pathogenic variants that delete the homeodomain than with missense or deletion pathogenic variants that include the paired domain. No genotype-phenotype correlation for the hearing loss in WS1 has been found.
PAX3 partial- or whole-gene deletions. There appears to be no discernable difference in the severity associated with whole- or partial-gene deletions and the clinical spectrum reported for small intragenic PAX3 pathogenic variants [Milunsky et al 2007].
PAX3 and MITF double heterozygotes (Waardenburg syndrome type I and Waardenburg syndrome type II [WS2] combined phenotype). Yang et al [2013] reported a family in which one parent had WS1 due to a heterozygous pathogenic variant in PAX3 and the other parent had WS2 (see Differential Diagnosis) due to a heterozygous pathogenic variant in MITF. Their child was heterozygous for both pathogenic variants and had significantly more pigmentary findings (i.e., white forelock, white eyebrows/eyelashes, and leukoderma) than either parent.
Penetrance
WS1 showed penetrance of at least 85% [Preus et al 1983] before the advent of molecular testing. Careful examination of individuals identified on the basis of pedigree analysis as having a PAX3 pathogenic variant usually reveals subtle findings (minor criteria). Hence, those individuals with an affected first-degree relative should be examined closely as the penetrance is likely almost complete.
Prevalence
It is difficult to quote a figure for the prevalence of WS1 without population-based molecular analysis. The prevalence figures vary from 1:20,000 to 1:40,000, accounting for approximately 3% of congenitally deaf children [Tamayo et al 2008].
Differential Diagnosis
Waardenburg syndrome type I (WS1) needs to be differentiated from other causes of congenital, non-progressive sensorineural hearing loss (see Deafness and Hereditary Hearing Loss Overview) and from other forms of Waardenburg syndrome.
Waardenburg syndrome type II (WS2). WS1 is distinguished from WS2 by the presence in WS1 of lateral displacement of the inner canthi (dystopia canthorum). If the average W index across a family is less than 1.95, the diagnosis is WS2. Sensorineural hearing loss and heterochromia iridum are the two most characteristic features of WS2. Both are more common in WS2 than WS1. White forelock and leukoderma are both more common in WS1 than in WS2 (see Table 3).
- MITF (OMIM 156845). Heterozygous MITF pathogenic variants [Yang et al 2013] have been described in approximately 10%-20% of individuals with WS2. MITF pathogenic variants have also been identified in individuals with Tietz syndrome (deafness with uniform hypopigmentation) [Léger et al 2012].
- SOX10 (OMIM 602229). Heterozygous SOX10 single-nucleotide variants [Iso et al 2008] and deletions [Bondurand et al 2007, Brezo et al 2014] have been described in about 15% of individuals with WS2. Chen et al [2010] indicated that SOX10 pathogenic variants occurred with a frequency similar to MITF pathogenic variants in individuals with WS2 of Chinese ancestry. Temporal bone abnormalities (specifically bilateral agenesis or hypoplasia of the semicircular canals with a cochlear deformity and enlarged vestibule) are also found in individuals with SOX10 pathogenic variants [Elmaleh-Bergès et al 2013]. Zhang et al [2012] and Chaoui et al [2011] performed functional analysis of SOX10 pathogenic variants. A frameshift variant showed a dominant-negative effect on wild type SOX10, leading to faster protein decay, possibly resulting in a milder WS2 phenotype [Zhang et al 2012].
- SNAI2. Biallelic SNAI2 pathogenic variants were reported in two individuals with features overlapping WS2 (OMIM 608890)
Table 3.
Comparison of Clinical Features in WS1 and WS2
| Clinical Finding | % of Affected Individuals | |
|---|---|---|
| WS1 | WS2 | |
| Sensorineural hearing loss | 47%-58% | 77%-80% |
| Heterochromic irides | 15%-31% | 42%-54% |
| Hypoplastic blue irides | 15%-18% | 3%-23% |
| White forelock | 43%-48% | 16%-23% |
| Early graying | 23%-38% | 14%-30% |
| Leukoderma | 22%-36% | 5%-12% |
| High nasal root | 52%-100% | 0%-14% |
| Medial eyebrow flare | 63%-73% | 7%-12% |
Based on Liu et al [1995], Pardono et al [2003], Tamayo et al [2008]
Waardenburg syndrome type IV (WS4). Individuals having a rare combination of pigmentary abnormalities, hearing loss, and Hirschsprung disease have WS4 [Jan et al 2008] caused by pathogenic variants in one of the following genes: EDNRB (OMIM 131244), EDN3 [Ohtani et al 2006] (OMIM 613265), or SOX10 [Bondurand et al 2007, Sznajer et al 2008] (OMIM 602229).
See Waardenburg syndrome: OMIM Phenotypic Series to view genes associated with this phenotype in OMIM.
Piebaldism (OMIM 172800). Piebaldism has some pigmentary features in common with Waardenburg syndrome. A white forelock is commonly seen along with absent pigmentation of the medial forehead and eyebrows. Absent pigmentation of the chest, abdomen, and limbs is also common. A characteristic feature is hyperpigmented borders surrounding the unpigmented areas. Heterochromia irides and sensorineural deafness are rarely described. This disorder has shown genetic heterogeneity with dominant loss-of-function variants/whole-gene deletions described involving the KIT proto-oncogene. Heterozygous pathogenic variants in SNAI2 have also been implicated as an etiology in some individuals with piebaldism [Sánchez-Martín et al 2003].
Management
Evaluations Following Initial Diagnosis
The following are appropriate:
- Audiology evaluation
- Consultation with a clinical geneticist and/or genetic counselor
Treatment of Manifestations
Management of the hearing loss associated with WS1 depends on its severity (see Deafness and Hereditary Hearing Loss Overview). Cochlear implantation has been successful in individuals with WS [Amirsalari et al 2012, de Sousa Andrade et al 2012, Koyama et al 2016].
Surveillance
The hearing loss in WS1 is typically non-progressive. Hence, repeat audiogram would usually not be necessary.
Evaluation of Relatives at Risk
It is appropriate to evaluate at-risk relatives of an affected individual to allow early screening of those at risk for hearing loss. Evaluations can include:
- Molecular genetic testing if the pathogenic variant in the family is known;
- Physical examination for the clinical features of WS1 and audiology evaluation if the pathogenic variant in the family is not known.
See Genetic Counseling for issues related to testing of at-risk relatives for genetic counseling purposes.
Pregnancy Management
Folic acid supplementation in pregnancy has been recommended for women at increased risk of having a child with WS1, given the possibly increased risk of neural tube defects in association with WS1 [Fleming & Copp 1998]; however, no human studies have addressed the ideal dose of folic acid to be used during pregnancy.
Therapies Under Investigation
Search ClinicalTrials.gov in the US and EU Clinical Trials Register in Europe for access to information on clinical studies for a wide range of diseases and conditions. Note: There may not be clinical trials for this disorder.