Ebstein Anomaly
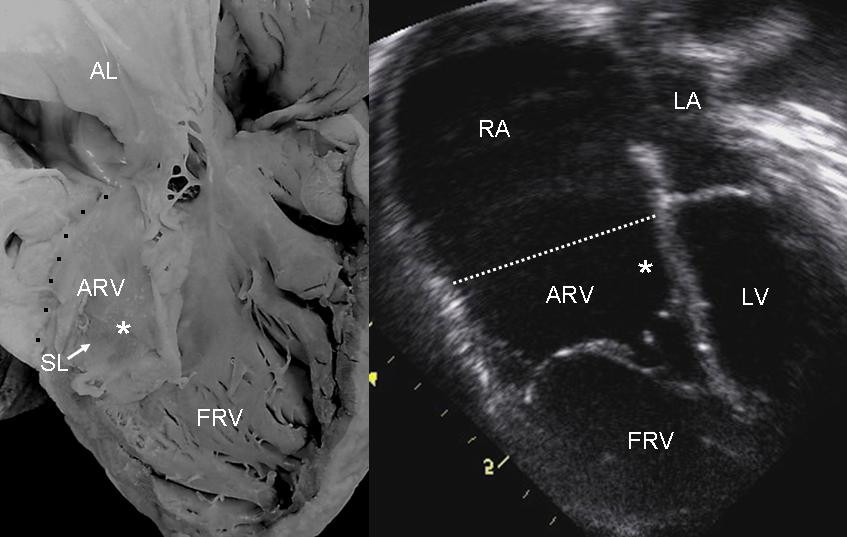
Description
Ebstein anomaly is characterized by downward displacement of variable severity of the tricuspid valve into the right ventricle. The valve leaflets may be dysplastic, and a variable portion of the proximal part of the right ventricle is in continuity with the right atrium ('atrialized'), because of the abnormally positioned tricuspid valve. The severity of this defect includes a spectrum ranging from severe disturbance in fetal and neonatal life to virtually asymptomatic survival to adult life. Associated extracardiac anomalies in the setting of chromosomal or mendelian disorders occur in about 20% of patients with Ebstein anomaly. Nonsyndromic Ebstein anomaly can occur as a sporadic or a familial defect (summary by Digilio et al., 2011).
Clinical FeaturesGouffault et al. (1960) found Ebstein malformation in 1 sib and a comparable deformity of the mitral valve in a sister. The same combination of Ebstein anomaly in 1 sib and comparable mitral anomaly in another was apparently present in the family reported by Yamauchi and Cayler (1964).
Gueron et al. (1966) described a brother and sister with Ebstein anomaly, a congenital malformation of the heart that consists of downward placement of the tricuspid valve such that part of the right ventricle becomes incorporated into the pretricuspid chamber. Associated deformity of the tricuspid leaflets and defect of the atrial septum are frequent.
Donegan et al. (1968) found Ebstein anomaly in a 6-year-old boy and his maternal uncle.
The Ebstein anomaly predisposes to right bundle branch block, preexcitation, and an increased risk of sudden cardiac death. Atrial fibrillation occurs in about one-third of patients with Ebstein anomaly. Pierard et al. (1985) reported atrial standstill in father and son with Ebstein anomaly.
McIntosh et al. (1992) diagnosed severe Ebstein anomaly prenatally in 2 sisters born of Sri Lankan parents who were first cousins once removed.
Digilio et al. (2011) studied 44 consecutive patients with Ebstein anomaly presenting to 2 pediatric cardiology centers. In 12 (27%) of the 44 patients, Ebstein anomaly was part of a syndrome, and 7 of those patients were diagnosed with distinct disorders, including CHARGE syndrome (214800) in 2, and VACTERL association (see 192350), Noonan syndrome (163950), Kabuki syndrome (147920), Holt-Oram syndrome (142900), and Cornelia de Lange syndrome (see 122470) in 1 each. Of the 32 patients with nonsyndromic Ebstein anomaly, 10 (31%) had additional congenital heart defects (CHDs), including 7 atrial septal defects, 2 ventricular septal defects, 2 pulmonary stenoses, 1 dextrocardia, 1 aortic coarctation, and 1 patent ductus arteriosus; 1 of the patients with ASD also had a Wolff-Parkinson-White cardiac conduction anomaly (WPW; see 194200). The remaining 22 patients had Ebstein defect alone; 1 of these patients also had WPW. Familial recurrence of CHD was seen in 1 family, in which a mother with nonsyndromic Ebstein anomaly had a son with persistence of left ventricular noncompaction (see 604169).
CytogeneticsIn 2 unrelated patients with Ebstein anomaly and other malformations, de Lonlay-Debeney et al. (1998) described 2 distinct rearrangements of the long arm of chromosome 11.
In a study of 44 consecutive patients with Ebstein anomaly, Digilio et al. (2011) performed standard chromosome analysis and array CGH in the 12 patients in whom the anomaly was part of a syndrome, and identified chromosomal anomalies in 3 of them: a 1p36 deletion (see 607872) in association with an Xpter-Xp22.3 duplication (see 300830), an 8p23.1 deletion, and a deletion of 18q21.3-qter (see 601808).
Molecular GeneticsIn 28 patients with nonsyndromic Ebstein anomaly, Digilio et al. (2011) screened the candidate genes GATA4 (600576) and NKX2.5 (600584), but did not find any mutations.
Animal ModelCanine tricuspid valve malformation (CTVM) is morphologically similar to Ebstein anomaly. Andelfinger et al. (2003) noted that familial occurrence of CTVM had been described, although most cases appeared to be sporadic. They studied 3 purebred Labrador retriever kindreds enriched for CTVM. Pedigree analysis indicated that CTVM segregated as an autosomal dominant trait with reduced penetrance. Genomewide linkage analysis in 1 kindred identified a CTVM susceptibility locus on chromosome 9 with a maximum multipoint lod score of 3.33. The 2 additional kindreds showed a conserved disease haplotype, indicating a founder effect in apparently unrelated Labrador retriever kindreds. The critical region of canine chromosome 9 contained a syntenic group of genes that has its human counterpart on 17q12-q23. Andelfinger et al. (2003) pointed to several possible candidate genes on 17q: TBX2 (600747) on 17q23, which plays a pivotal role in chamber differentiation and atrioventricular canal formation; TOB1 (605523) on 17q21, which is an important transmitter in the ERBB2 (164870) cascade involved in cardiac muscle and valve formation; and FZD2 (600667) on 17q21.1, which is a receptor in the Wnt-dishevelled signal transduction cascade expressed during cardiac morphogenesis.