Gitelman Syndrome
A number sign (#) is used with this entry because of evidence that Gitelman syndrome (GTLMNS) is caused by homozygous or compound heterozygous mutation in the (SLC12A3; 600968) on chromosome 16q13.
DescriptionGitelman syndrome is an autosomal recessive renal tubular salt-wasting disorder characterized by hypokalemic metabolic alkalosis with hypomagnesemia and hypocalciuria. It is the most common renal tubular disorder among Caucasians (prevalence of 1 in 40,000). Most patients have onset of symptoms as adults, but some can present in childhood. Clinical features include transient periods of muscle weakness and tetany, abdominal pains, and chondrocalcinosis (summary by Glaudemans et al., 2012). Gitelman syndrome is sometimes referred to as a mild variant of classic Bartter syndrome (607364).
For a discussion of genetic heterogeneity of Bartter syndrome, see 607364.
Clinical FeaturesIt has been proposed that Bartter syndrome, defined generically as an autosomal recessive disorder featuring hypokalemic metabolic alkalosis with salt wasting, is a heterogeneous entity with at least 2 subsets, Gitelman syndrome and 'true Bartter syndrome.' Simon et al. (1996) stated that Gitelman syndrome refers to the numerically predominant subset of patients with hypokalemic alkalosis in conjunction with hypocalciuria and hypomagnesemia, whereas true Bartter syndrome refers to patients with normal or hypercalciuria and typically normal magnesium levels. True Bartter patients usually present under the age of 5 years with signs of intravascular volume depletion, whereas Gitelman syndrome patients typically present at older ages without overt hypovolemia. Nevertheless, the overlapping features of these disorders has resulted in considerable confusion and controversy regarding their classification, with many patients with features of Gitelman syndrome being labeled as having Bartter syndrome.
Gitelman et al. (1966) reported 2 affected sisters who were the offspring of parents related as half first cousins once removed. They had experienced occasional mild episodes of muscle weakness and had suffered for many years from a chronic dermatitis characterized by thickening with a purple-red hue. Erythema of the skin is a feature of experimental magnesium depletion in the rat. Spencer and Voyce (1976) reported 3 affected sibs. Symptoms were precipitated by nonspecific illness and consisted mainly of tetany. A depressed creatinine clearance in the oldest sib (aged 19 years) suggested renal damage from hypokalemia. Long-term correction of the potassium deficiency is warranted.
Zarraga Larrondo et al. (1992) described a 33-year-old woman who presented with hypokalemia-hypomagnesemia associated with renal potassium and magnesium wasting. Her mean 24-hr urinary calcium excretion was strikingly low despite normocalcemia, normal creatinine clearance, and normal serum parathyroid hormone and calcitriol. In response to intravenous furosemide, the patient showed significant increments in sodium, chloride and magnesium excretion as well as abolition of hypocalciuria. The dissociation of renal calcium transport from magnesium transport together with exaggerated natriuresis after furosemide suggested the presence of a defect in the distal tubule rather than in the loop of Henle. The patient's symptoms included paresthesia and carpopedal spasms. She had been hospitalized at the age of 23 for tetanic convulsions which were interpreted as periodic hypokalemia. She often felt tired and had muscle cramps provoked by physical exertion. She had not been treated with diuretics, antirheumatics or hormones, and denied laxative abuse or diarrhea.
Hisakawa et al. (1998) identified 25 cases of an association between Bartter syndrome and chondrocalcinosis (118600) that were reported between 1978 and 1998. Hypomagnesemia had been associated with chondrocalcinosis. Because all of the reports of an association with Bartter syndrome referred to hypomagnesemia and hypocalciuria, Hisakawa et al. (1998) suggested that these might be cases of Gitelman syndrome, not 'true' Bartter syndrome. Hisakawa et al. (1998) described a 45-year-old Japanese woman, treated for Bartter syndrome for 14 years, who presented with numbness of her extremities and polyarthralgia. It was concluded that she had Gitelman syndrome with chondrocalcinosis, and treatment with spironolactone and magnesium supplementation was effective. Previously reported cases of 'Bartter syndrome' with chondrocalcinosis were tabulated.
Gitelman syndrome is widely described as a benign or milder variant of Bartter syndrome. However, Pachulski et al. (2005) described a patient with this disorder who presented with presyncope coincident with long runs of ventricular tachycardia. The otherwise well 39-year-old woman took no medications. Serum potassium and magnesium levels were very low. An electrophysiologic study was negative for inducible, sustained ventricular tachycardia; however, the patient continued to have ventricular tachycardia with presyncope despite aggressive potassium and magnesium supplementation. The patient was managed with amiodarone and an implanted defibrillator to guard against a potential breakthrough of sustained ventricular arrhythmia. Pachulski et al. (2005) pointed to reports suggesting that about half the patients with Gitelman syndrome have QTc prolongation. Although Gitelman syndrome is described as an asymptomatic or benign disorder with characteristic electrolyte abnormalities, most reports of clinical series document presyncope, vertigo, ataxia, and blurred vision. Patients, particularly those with prolonged QTc, should be investigated for ventricular arrhythmia.
Ng et al. (2006) reported a 22-year-old Chinese man with Gitelman syndrome. He reported periodic paralysis since age 11 years. The episodes usually developed after strenuous exercise and lasted 2 to 3 days with spontaneous resolution. Laboratory studies showed decreased serum potassium, decreased serum magnesium, and low calcium excretion. Genetic analysis identified compound heterozygosity for mutations in the SLC12A3 gene (600968.0013; 600968.0014). His unaffected parents were heterozygous for each of the mutations. The patient's sister, who had the same genotype, had decreased serum potassium and decreased calcium excretion, but did not have any clinical symptoms. Ng et al. (2006) noted the intrafamilial variability and suggested a gender effect.
Heterozygous Mutation Carriers
Among relatives of patients with Gitelman syndrome, Fava et al. (2008) identified 35 individuals who were heterozygous carriers of mutations in the SLC12A3 gene. Compared to unrelated matched controls, the heterozygous carriers had markedly lower blood pressure (systolic 103.3 vs 123.2 mm Hg; diastolic 62.5 vs 73.1 mm Hg; p less than 0.001). Although there were no significant differences between the groups in plasma concentration or urinary excretion rate of electrolytes, heterozygous mutation carriers had slightly higher fasting plasma glucose concentrations. Overall, the phenotype of heterozygous mutation carriers was similar to patients being treated with low-dose thiazide diuretics, suggesting that they are partially protected from hypertension through partial genetic loss of function of the SLC12A3 transporter.
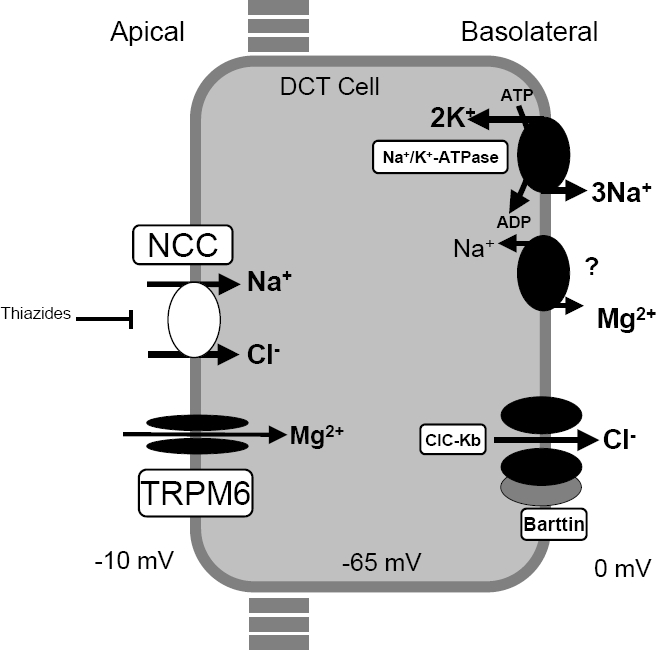
MappingAn attractive candidate gene for one form of Bartter syndrome is the thiazide-sensitive Na-Cl cotransporter of the distal convoluted tubule (SLC12A3; 600968), which is believed to be the principal mediator of sodium and chloride reabsorption in this segment of the nephron, accounting for a significant fraction of net renal sodium reabsorption. This cotransporter is the target of thiazide diuretics, one of the major classes of agents used in the treatment of hypertension. Simon et al. (1996) demonstrated complete linkage of Gitelman syndrome to the thiazide-sensitive Na-Cl cotransporter gene on 16q13.
Molecular GeneticsIn patients with Gitelman syndrome, Simon et al. (1996) identified a wide variety of nonconservative mutations in the SLC12A3 gene consistent with loss-of-function alleles (600968.0001). Many of their patients were genetic compounds and this, together with the finding of independent mutant alleles in different branches of kindreds, suggested to the authors that mutant alleles are not rare in the population. Moreover, the same conclusion was suggested by the finding that consanguineous marriage is not prominent in Gitelman syndrome kindreds. The prevalence of heterozygotes may be at least 1% in Swedish and Italian populations. A puzzling finding was the high proportion of affected offspring of heterozygous parents. After excluding index cases, they found that 22 of 33 offspring of heterozygous parents had Gitelman syndrome, far more than the expected 8 affected subjects. Whether this was the result of ascertainment bias or segregation distortion remained to be determined. Simon et al. (1996) speculated that these mutant alleles lead to reduced sodium chloride reabsorption in the heterozygotes, potentially protecting against development of hypertension. They noted that identification of specific mutations causing Gitelman syndrome permits testing of these hypotheses by identifying cohorts of heterozygous carriers and comparing their blood pressures and responses to pharmacologic intervention to those of their homozygous wildtype sibs or other controls. Subjects with unexplained hypokalemia or hypokalemia complicating drug therapy may be carrying Gitelman mutations. The potential for DNA diagnosis of this disorder is indicated by the fact that at initial presentation some patients with this disease are incorrectly believed to be diuretic abusers or to have bulimia (607499). Indeed, one of the patients of Simon et al. (1996) was committed to a locked psychiatric ward and it was only when her hypokalemia persisted for 2 weeks in this setting that a proper diagnosis was made.
Jeck et al. (2000) described 3 unrelated patients presenting with the typical laboratory findings of Gitelman syndrome. Mutation analysis in these 3 patients revealed no abnormality in the SLC12A3 gene. Instead, all patients were found to carry previously described mutations in the CLCNKB (602023) gene: 2 were homozygous for complete deletion of the gene (602023.0006) and 1 was homozygous for an A-G substitution at the splice acceptor site of intron 7 (602023.0007). Review of the medical histories revealed manifestation of the disease within the first year of life in all cases. Clinical presentation included episodes of dehydration, weakness, and failure to thrive, much more suggestive of classic Bartter syndrome type 3 (607364) than of Gitelman syndrome. The coexistence of hypomagnesemia and hypocalciuria was not present from the beginning. In the follow-up, a drop of both parameters below normal range was a consistent finding reflecting a transition from classic Bartter syndrome to Gitelman syndrome phenotype. Jeck et al. (2000) suggested that the phenotypic overlap may indicate a physiologic cooperation of the apical thiazide-sensitive Na-Cl cotransporter and the basolateral chloride channel for salt reabsorption in the distal convoluted tubule.
Animal ModelYang et al. (2010) found that mice with a homozygous truncating mutation in the Slc12a3 gene (S707X) showed features of Gitelman syndrome, including relative hypotension with increased plasma renin activity, increased aldosterone levels, hypokalemia, hypomagnesemia, hypocalciuria, and metabolic alkalosis. There was markedly reduced Slc12a3 mRNA and protein expression in the kidneys, which was primarily due to nonsense-mediated mRNA decay. The later distal convoluted tubules of mutant mice showed increased cell volume, but cell structure was similar to wildtype. The distal and cortical collecting tubules had increased expression of the epithelial sodium channel Scnn1b (600760), calcium channels Trpv5 (606679) and Trpv6 (606680), and potassium channels Romk1 (KCNJ1; 600359) and Kcnma1 (600150), which was also observed in a renal biopsy from a human patient with GS. These changes indicated adaptive changes in channel expression, which may contribute to electrolyte imbalances observed in the disorder.